e-ISSN: 2347-7857 p-ISSN: 2347-7849
e-ISSN: 2347-7857 p-ISSN: 2347-7849
Department of Pharmaceutics and Research, Swamy Vivekanandha College of Pharmacy, Tiruchengode–637205, Tamil Nadu, India.
Received date: 28 September 2013 Accepted date: 01 November 2013
Visit for more related articles at Research & Reviews: Journal of Pharmaceutics and Nanotechnology
In the past few decades, considerable attention has been focused on the development of new drug delivery system (NDDS). The NDDS should ideally fulfill two prerequisites. Firstly, it should deliver the drug at a rate directed by the needs of the body, over the period of treatment. Secondly, it should channel the active entity to the site of action. Now a days vesicular drug delivery system plays an important role as it is targeting type of drug delivery system which includes liposomes ,niosomes pharmacosomes and virosomes. Vesicular drug delivery systems delay drug elimination of rapidly metabolizable drugs, and function as sustained release systems. Niosomes are multilamellar or unilamellar vesicles wherein an aqueous solution of solute(s) is enclosed in highly ordered bilayer made up of non-ionic surfactant with or without cholesterol and dicetyl phosphate and exhibit behavior similar to liposomes. Niosomes of Zidovudine were prepared by ether injection method by varying ratios of span 80 and span 20 with cholesterol in ratio of 1:1,2:1and 3:1.and dicetyl phosphate added to the formulation to prevent aggregation of vesicles .the prepared vesicles were characterized for their size ,entrapment efficiency leakage studies ,osmotic shock and invitro drug release profile and the niosomes were characterized by their entrapment efficiency and invitro release profile and the niosomes with span and cholesterol in ratio of 2:1 had high drug release profile were found to be best formulations and they increase the therapeutic effectiveness of Zidovudine and prolong the therapeutic action of drug.
Niosomes, Zidovudine, zeta potential, dicetyl phosphate
In the past few decades, considerable attention has been focused on the development of new drug delivery system (NDDS). The NDDS includes sustained release and controlled release drug delivery systems. Vesicular drug delivery system plays a major role in modeling biological membranes, and in the transport and targeting of active agents. Niosomes are multilamellar or unilamellar vesicles wherein an aqueous solution of solute(s) is enclosed in highly ordered bilayer made up of non-ionic surfactant with or without cholesterol and dicetyl phosphate and exhibit behavior similar to liposomes. Niosomes are prepared by various methods and in this work Zidovudine niosomes were prepared by ether injection method using varying ratios of span 80 and span20 and they were evaluated for their particle size ,entrapment efficiency osmotic shock ,drug leakage studies, zeta potential measurement and they were characterized by their invitro studies and results indicated that niosomes with span 80 and span 20 and cholesterol in ratio of 2:1 showed high drug release rate and the niosomes prepared by this method have high therapeutic action and these are needed for prolonged action for longer time [1,2].
Zidovudine is an anti- HIV drug used in the treatment of human immuno deficiency viral treatment .Zidovudine which is having shorter biological half life and low bioavailability it is modified into noisomes so that its bioavailability can be increased.The major objective of the present investigation is to develop niosomes of Zidovudine using ether injection method
Zidovudine was a gift sample from auribindo labs Hyderabad ,span 80 and span 20, cholesterol ,dicetyl phosphate and diethyl ether were of analytical grade and were used as received .
Preparation Of Zidovudine Niosomes - By Ether Injection Method
Cholesterol and non-ionic surfactants i.e.Span80 & Span20 were taken in prescribed ratio (1:1, 1:2 &1:3) in a 50 ml beaker. The mixture was dissolved in diethyl ether and the solution was slowly injected into a beaker containing Zidovudine in phosphate buffer saline (PBS) pH 6.8.The temperature maintained during the injection was 40-600C. The differences in temperature between phases cause rapid vaporization of ether resulting in spontaneous vesiculation [3].
Based on the entrapment efficiency the formulation which is having higher entrapment were selected for the two surfactants i.e. Span 80 & Span 20 and dicetyl phosphate was added at a constant ratio i.e. 5mg and then evaluated for other parameters
Evaluation of Different Batches Of Niosomes
Morphology and Particle Size
The prepared niosomal vesicles were observed under microscope and observed for their shape and size.First they were observed under low power and then high power and their size was measured. And their size was measured using scanning electron microscopy(SEM) Small amount of sample of niosomes suspension was taken in cover slip on the specimen stub .it was then coated with carbon and then with gold vapor using Hitachi vacuum evaporator ,model HITACHI S 5 GB. The samples were viewed under scanning electron microscope, which was operated at 10 kilo volts and then photographed [4].
Percentage Encapsulation of the Drug
Zidovudine loaded niosomes were first centrifuged at 2000rpm for 45min and then the supernatant was collected and further dilutions were carried out as required and the absorbance was measured spectrophotometrically at 266nm.and the amount of drug unentrapped was identified and from that the total amount of drug entrapped was known [3]. This was carried out to find out the percentage of drug encapsulated in the niosomes by using the formula,
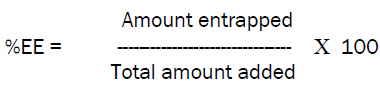
Osmotic Shock
Effect of osmotic shock was determined by measuring the change in the vesicle sizes followed by incubating the vesicular suspension in media of different tonicity including hypertonic, hypotonic and Phosphate buffer pH 6.8. The vesicular suspension was incubated in this media for three hours following with which change in vesicle size was measured [5].
Drug Leakage Studies from Vesicles
Vesicles stability with respect to drug leakage and drug degradation up on storage was studied at refrigeration temperature(4°C),room temperature(25°C)high temperatures (37°C) for a period of one month on niosomes samples containing a known amount of Zidovudine ,contained in light resistant containers .samples were with drawn at weekly intervals and entrapment was determined [6].
Zeta Potential Measurement
Zeta potential values of niosomes were determined using zetameter3.0.vesicular dispersions were diluted to 1in 100 with phosphate buffer pH 6.8.The dilute niosomal dispersion was charged in zeta meter cell and voltage was set at 50v to optimize the measurement of electrophoretic mobility. The charge of vesicles and average zeta potential were obtained directly [5].
In Vitro Release Pattern of Zidovudine Niosomes
The niosomal preparation was taken in dialysis tube, which acts as donor compartment. dialysis tube was placed in beaker containing 250ml of phosphate buffer of pH 6.8, which acts as receptor compartment. The temperature of the medium maintained at 37±1ºC and the medium agitated at moderate speed using magnetic stirrer. .1ml of aliquot sample was taken and made up to 10ml with PBS.1ml of diffusion medium was replaced after every with drawal of sample so that volume of diffusion medium was maintained .the collected samples were analyzed at 266nm [6].
Niosomes of Zidovudine were prepared by ether injection method using cholesterol and various ratios of surfactants like Span 80 & Span 20 and dicetyl phosphate, a charge inducing substance was added to the best formulations based on the percent of drug entrapped to prevent aggregation of vesicles.
Niosomes were evaluated for their morphology, vesicle size, entrapment efficiency, drug leakage, osmotic shock, zeta potential and invit o release profile. The compatability studies reveal that there was no interaction between drug and excepients and further studies can be carried out.
Morphological studies reveal that the niosomes were discrete, spherical in shape and they were in size range of 0.5μm- 2.5μm with an average size of 1.5μm.The vesicle size of the best formulations ES2 and ES7 is presented in figures.
The percentages of drug entrapped in niosomes is given in Table No:3.The Percentage of drug entrapped in the formulations ES1 to ES6 ranged between 82.5% to 88.4%.The higher percentage of drug entrapped was found to be with ES2 and lower with ES6.Based on the entrapment efficiency the two best formulations ES2 and ES5 were further treated with dicetyl phosphate (DCP).After treatment with DCP the entrapment efficiency of the two formulations did not show significant change in entrapment efficiency
Effect of osmotic shock on niosomes is presented in Table No: 5 .All vesicular suspensions incubated with hypertonic media (1.5 % w/v NaCl) for 3hrs shrinked completely. This was observed with all the formulations. Similarly, incubation of vesicular suspension in a hypotonic media (0.5 % w/v NaCl) recorded an increase in their vesicle size. There was a significant increase as compared to the vesicular dispersion in PBS pH 6.8. However, no significant change in vesicle size was observed with vesicles incubated in normal saline (0.9 % w/v NaCl).
The percent drug retention Vs storage at 7, 14, and 21 & 28 days at refrigeration temperature (4º±1ºC), room temperature (25º±1ºC) and high temperature (37º±1ºC) is presented in Table No: 6. Niosomes have shown a fairly high retention of the drug inside the vesicles at a refrigeration temperature up to a period of one month (ïÃâû90%). While, storage at room temperature lead to a substantial loss (ïÃâû20 %) and storage at high temperature lead to increase in loss (ïÃâû 40 %) of the drug from the niosomes at the end of one-month period.
The zeta potential of the best formulations was measured and they are reported. The average zeta potential value measured for normal niosomes was found to be -3.78mv.Normally the vesicles prepared with out inclusion of charge inducer should possess negative zeta potential due to the adsorption of counter ions.
When a charge inducing substance was added then the zeta potential value was high i.e.-2.89mv, which shows that when the negative charge inducer was added zeta potential value increases and this charge inducer added prevent aggregation of vesicles. The presence of surface charge in vesicular dispersions is important for prevention of aggregation of the vesicles.
In Vitro Evaluation of Zidovudine Niosomes
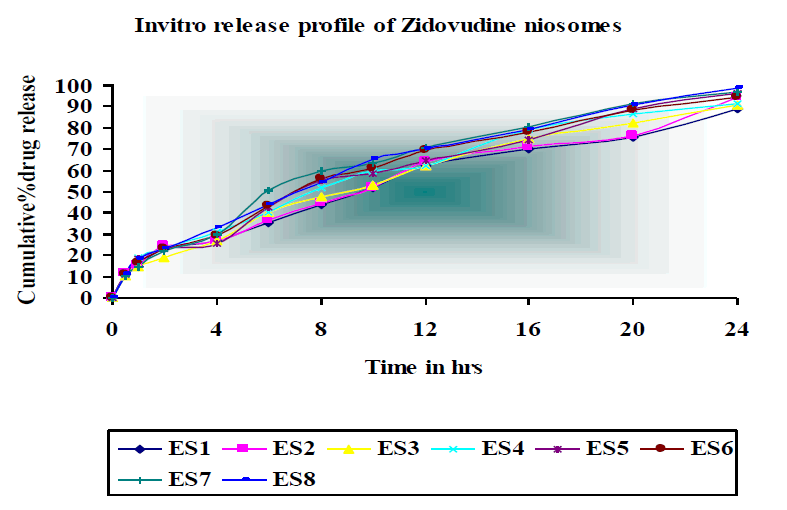
In vitro drug release was studied for all the batches of niosomes. The studies were performed up to 24 hr for all the batches. The cumulative % release of Zidovudine from various formulations by ether injection method at 24 hr was found to be 94% - 98%.
The formulation ES6 shows high percentage of drug release and ES4 show lower percentage of drug release. Almost constant drug release was observed in all formulations indicating zero order release pattern. This is indicated by the linearity in regression value when plotted with cumulative percentage release Vs time curve.
The present study aimed at developing a targeted drug delivery system of Zidovudine, an anti-HIV drug and evaluating for various Physico-chemical characteristics and invitro dissolution study .
Niosomes of Zidovudine were prepared by ether injection method using cholesterol with varying ratios of surfactants like Span 80 and Span 20 and the best formulations based on the percent of drug entrapped were selected. Dicetyl phosphate, a charge inducing substance was added to the selected formulations to prevent aggregation of vesicles.
Morphological studies reveal that the niosomes were discrete, yellow in colour with size range of 0.5-2.0μm.
The percentage of drug encapsulated was found to be in the range of 84.5% to 88.7%. Niosomes with Span 80 showed higher percentage of drug entrapment compared to niosomes with Span 20 due to high lipophilic character of Span 80.
The Osmotic shock study indicates that, there was significant change in vesicle size which when incubated in hypertonic or hypotonic media, however showed no change in normal saline. The increase in vesicle size may be due to cholesterol present in the vesicles which may cause rigidization.
Drug leakage studies were carried out at varying temperatures i.e. at refrigeration, room temperature and high temperatures and it was found that the niosomes are stable at refrigeration temperatures.
Zeta potential measurements were carried out to investigate surface characteristics of the niosomal vesicles. The niosomal vesicles normally have a negative zeta potential. The presence of surface charge in vesicular dispersions is important and when dicetyl phosphate a negative charge inducing substance was added the zeta potential value is high and presence of charge inducing substance prevents aggregation of niosomes.
Invitro release pattern of niosomal formulations were observed and the mean cumulative percent drug release of Zidovudine was found to be 94%-98%. The cumulative percentage of drug released was high for Span 20 based formulations. This difference in release rate was due to its low lipophilicity than Span 80 and almost constant drug release was observed in all formulations.
The Zidovudine niosomes were prepared and they were evaluated for their physicochemical characteristics and their size range was found to be about 0.5μ-2μm and the Zidovudine niosomes were stable at refrigeration temperatures and high percent of drug retention was found in normal saline solution or in phosphate buffer and the drug release was found to be 94-98% with constant drug release and when dicetyl phosphate was added there was no aggregation and they enhance the therapeutic effectiveness of the Zidovudine niosomal formulations.
The present study conclusively states that the niosomal formulations of Zidovudine can be considered advantageous over the conventional drug deliveries like Zidovudine tablets, since the niosomes provide zero-order release kinetics which is essential for prolonged action of the drug. It can be further stated that where the drug therapy requires longer duration of treatment as in HIV infections the Zidovudine niosomal formulations can be considered ideal.