e-ISSN: 2347-7857 p-ISSN: 2347-7849
e-ISSN: 2347-7857 p-ISSN: 2347-7849
Amrutha C*, Saujani Kamble, Ravikiran S, Manjunath Nasi, Manu N
Department of Pharmacognosy, Birla Institute of Technology, Bengaluru, India
Received: 07-Aug-2021, Manuscript No. JPN-21-001- PreQc-22; Editor assigned: 10-Aug- 2021, Pre QC No. JPN-21-001- PreQc- 22(PQ);Reviewed: 24-Aug- 2021, QC No. JPN-21-001-PreQc- 22; Revised: 22-Jul-2022, Manuscript No. JPN-22-001- PreQc-22(R); Published: 09-Sep- 2022, DOI: 10.4172/23477857.10.3.001.
Visit for more related articles at Research & Reviews: Journal of Pharmaceutics and Nanotechnology
Nanotechnology is an important field of modern research dealing with design, synthesis, and manipulation of particle structures ranging from approximately 1-100 nm. Metal nanoparticles such as Nanotechnology refers to the creation and utilization of materials whose constituents exist at the nanoscale; and, by convention, be up to 100 nm in size. It has the potential to revolutionize a series of medical and biotechnology tools and procedures so that they are portable, cheaper, safer, and easier to administer. In the present study, biosynthesis of silver nanoparticles and its activity on free radical scavenging were investigated. Silver nanoparticles were rapidly synthesized using leaf extract of Albizia lucidior and the formation of nanoparticles was observed within 4 hrs. The results recorded from UV–visible spectrum, Scanning Electron Microscopy (SEM), Zeta potential and particle Size analyzer support the biosynthesis and characterization of silver nanoparticles. Further, the antioxidant activity of synthesized silver nanoparticles shows effective activity silver nanoparticles were measured 18-25 nm.
Nanoparticles, weeds , Anti-oxidants
Ag, Au, Pt and Pd, are extensively applied in products that directly come in contact with the human body, such as household items like detergents, soaps, shampoos, cosmetic products, and toothpaste and they also find applications in the pharmaceutical and medical area [1-2]. Albizia is a genus of more than 160 species of mostly fast-growing subtropical and tropical trees and shrubs in the subfamily Mimosoideae of the family Fabaceae. The genus is pan tropical, occurring in Asia, Africa, Madagascar, America and Australia, but mostly in the Old-World tropics. In some locations, some species are considered weeds.
Several species of this genus such as A. julibrissin Durazz., A. lebbeck Benth., A. gummifera C.A. Sm., A. chinensis Merr., A. adianthifolia (Schumach) W. F. Wright, and A. procera (Roxb.) Benth have been widely used for the treatment ofmelancholia, insomnia, wounds, fever, abscesses, diabetes, headache, stomach-ache, diarrhoea, cough, rheumatism, snake bite, malaria, and parasitic infection in traditional and local medicine [3].
Photograph representing Albizia lucidior tree and leaves from Foundation for Revitalization of Local Health Traditions (FRLHT). Identification and authentication of plant material was done by Dr. N.M. Ganesh Babu, Assistant professor, heading centre for Herbal Gardens, Foundation for Revitalization (Figure 1).
Plant material and preparation
The fresh leaves of Albizia lucidi006Fr (Steud) I.C. Nielsen was collected Local Health Traditions (FRLHT), Bengaluru. The leaves were thoroughly washed 2-3 times in running water then with distilled water and was shade dried.
Proximate analysis
The proximate analysis determines the percentage of foreign particles present in the drug and authenticity of the selected material with respect to the literature survey [4]. The proximate analysis of the involved the total ash, alcohol extractive value and water extractive values which are found to be 5.525 ± 0.012, 12.36 ± 0.53 and 7.42 ± 0.52 respectively. Values are represented in terms Mean ± SEM of results conducted in triplicates (Table 1).
| Sl no | Standard parameters (%) | Albizia lucidior |
|---|---|---|
| 1 | Total Ash Value | 6.333 ± 0.012 |
| 2 | Alcohol Extractive Value | 9.3 ± 0.05 |
| 3 | Water Extractive Value | 11.23 ± 0.52 |
| 4 | Moisture content | 8.91 ± 0.05 |
Table 1. The Proximate Analysis values
Extraction of Albizia Lucidior analysis
The collected leaf powder of Albizia lucidior was stored in airtight containers until further studies. The Methanolic extract was prepared by using manually operated Soxhlet extractor according to the procedure mentioned in the literature (Figure 2). The dried leaf powder was packed in the thimble made from the filter paper such that it is completely immersed in the extraction solvent when kept in the siphoning tube (Table 2). The Methanol as solvent was used to extract the phytochemical constituents from the leaf powder of Albizia lucidior [5]. The stock extract was collected and subjected to rotary evaporator to retrieve the solvent and obtain a semi-solid extract which was further dried using incubator. The dried stock extract was preserved in an airtight container and stored in the refrigerator for further studies. The percentage of yield of extract was calculated and summarized in the results section.
| Sl no | Solvent | Odour | Color and nature | Percentage yield (%) |
|---|---|---|---|---|
| 1 | Methanol | Characteristic | Greenish and semi-solid | 23.135 ± 0.054 |
Table 2. Extraction details of Albizia lucidior.
Phytochemical details
Phytochemical screening of Albizia lucidior extract in methanolic solvent was carried out and results found that Alkaloids, Triterpenoids, Phenolic and tannins, Saponins and Flavonoids (Table 3).
| Sl no | Phytochemical constituents | Results |
|---|---|---|
| 1 | Carbohydrates | - |
| 2 | Glycoside | - |
| 3 | Alkaloids | + |
| 4 | Triterpenoids | + |
| 5 | Phenolic and tannins | + |
| 6 | Saponins | + |
| 7 | Proteins and amino acids | - |
| 8 | Flavonoids | + |
Table 3. Phytochemical constituents present in Albizia Lucidior methanolic extract.
Determination of total phenolic content
Phenolic compounds are very abundant in natural plants. These include Gallic acid, ellagic acid, Resveratrol, Caffeic acid, Ferulic acid, Kaempferol, Myricetin, anthocyanidin and many more. The estimation of the phenolic compounds is necessary to determine the amount of phenolic compound present in the selected plant material [6]. Phenolic compounds are estimated and represented as Gallic acid Equivalent, as Gallic acid is used a Standard phenolic compound (Figure 3). The percentage of Phenolic compound present in the methanolic extract of Albizia lucidior leaves were determined by Folin-Ciocalteu method. The calibration curve was plotted using Gallic acid as standard. TPC present in methanolic extract was expressed as 4.13 mg Gallic Acid Equivalent/gm of extract (Figure 3) [7].
Preparation of 0.01 mM AgNO3: 169.8 mg of AgNO3 was dissolved in 100 ml of distilled water.
Preparation of plant extract: 30 g of leaves were extracted with 300 ml of methanol using Soxhlet apparatus for about 7 days at 40ºC. The extraction was carried out, until solvent becomes colorless in siphoning tube. After cooling it was filtered through Whatman filter paper. 1 to obtain a methanolic extract of definite concentrations. The methanolic extract was transferred into tarred Petri plate, solvent was dried on hotplate at 35ºC, cool, store extract in refrigerator for further use [8]. The required concentration of the extract was dissolved in Milli Q water and used for green synthesis of silver nanoparticles.
Synthesis of Silver nanoparticles: For synthesis of silver nanoparticles, leaf extract pf 300 mg/ml was added to 0.01 mM AgNO3 solution 1:4 ratios and the mixture was stirred continuously for 4 H. The quick conversion of colour change from light or fluorescent green to brownish black colour showed the formation of silver nanoparticles (Figure 4).
Characterization of zinc oxide nanoparticles
UV visible: UV spectroscopy is type of absorption spectroscopy in which light of UV-Visible region (200-800 nm) is absorbed by the molecule. Absorption of ultra-violet radiations results in the excitation of the electrons from the ground state to higher energy state. The energy of the ultra-violet radiation that are absorbed is equal to the energy difference between the ground state and higher energy states (delta E=hf). UV-Visible spectroscopy is most widely used method that helps in the determination of concentration of analyse present in the given sample solution. It helps in the detection of impurities and detection of functional groups present in the sample [9]. It is used to detect the conjugation of the compounds. UV-Visible spectrophotometer consists of radiation source, monochromators, sample compartment, detector and recorder. UV absorption of zinc oxide nanoparticles which was synthesized using lemongrass essential oil was carried out over the range of 200-800 nm (Figure 5).
In this method, an electron beam is focused onto the sample surface when kept with properties of both particle and wave; hence an electron beam can be focused or condensed like an ordinary light). The beam is then scanned over the surface of the sample. The scattered electron from the sample is then fed to the detector and then to a cathode ray tube through an amplifier which is attached, where the images are formed, that gives the information of the sample. It comprises of a heated filament source of electron beam, condenser lenses, aperture, chamber for placing the sample, electron detector, amplifier and the image forming electronics. The SEM is an instrument that produces a large magnified image by using electrons instead of light to form an image [10]. Scanning Electron Microscopy (SEM) is most widely used to determine the surface characteristics of metals, ceramics, polymers, composites and biological materials for compositional analysis and topographic studies. This in vacuum by electromagnetic lenses (since the electrons possesses dual nature technique is extensively used in the analysis of metallic and ceramic inclusions, diffusion profiles in electronic components and inclusions of polymeric materials. Instrument specification: The TM3030Plus Scanning Electron Microscopy manufactured by Hitachi Ltd, Japan with power backup, computing and imaging facilities was used in the analysis. 15 to 120,000 × signal select observation mode 5kV/15kV, Back Scattered electrons/Secondary electron or Mix (30%BSE: 70%SE) detector with Conductor/Standard/Charge up Reduction modes. Maximum sample size 70 mm diameters. Auto start, Auto focus, Auto brightness/contrast [11]. Image size: 640 × 480 pixels as BMP, TIFF, JPEG, Auto micron marker. Turbomolecular pump. Image output in CD/DVD shown in Figure 6.
Zeta potential: The zeta potential of the nanoparticles was measured using Zetasizernano ZSP, Malvern Instrument Limited, USA. The nanoparticles were diluted with the ratio of 1:9 with distilled water and mixed for a minute and subjected for analysis (Figure 7).
Particle size determination
The particle size was measured by Malvern zeta sizer Nano ZS-90. Samples were prepared by diluting nanoparticles using sufficient quantity of HPLC water. The average diameter was determined from the particle size distribution (Figure 8) [12].
Evaluation of in-vitro anti-oxidant activity
Principle: DPPH (α, α-diphenyl-β-picrylhydrazyl) is stable free radical which scavenges when treated with antioxidant. DPPH assay method is a colorimetric assay where the reducing property of the drug is determined by the color change in the DPPH solution. The odd electron of nitrogen atom present in the DPPH Z*+AH=ZH+A solution is reduced by the hydrogen atom present in the treated antioxidant to the respective hydrazine [13]. This colorimetric assay is based on the measurement of the scavenging capacity of the compound. The delocalization of the electrons in the solution give rise to violet color, upon mixing with DPPH the free radical form N-H bond which leads to the formation of yellow colored solution (Figure 9).
Procedure
Reagents: Methanol, DPPH.
Standard Antioxidant: L- Ascorbic Acid (25-150 µg/ml)
Absorbance: 517 nm
Preparation of Methanolic DPPH: 2.36 mg of DPPH is dissolved in 100 ml of methanol to get 6*10-5 M Methanolic DPPH Solution, preserved in dark place.
Preparation of standard/extract concentration: 1 mg/ml Concentration of Standard Stock Solution is prepared by using methanol. Pipette out the Standard Solution to make 25, 50, 75, 100, 150 µg/ml of concentration [14].
Preparation of standard/sample
Sample mixture for determination: 1 ml of Standard/Sample is taken in calibrated test tube, add 3 ml of methanolic DPPH Solution and incubate in dark place for 30 mins. Methanol is used as Control. Absorbance of Standard, Sample, Control recorded at 517 nm using UV-Spectrophotometer (Table 4).
| Sl no | Concentration (µg/ml) | Meal | AL AgNP’s | Ascorbic acid |
|---|---|---|---|---|
| 1 | 50 | 69.24 ± 0.638 | 71.082 ± 0.152 | 96.336 ± 0.056 |
| 2 | 100 | 73.13 ± 0.110 | 75.341 ± 0.002 | 96.420 ± 0.025 |
| 3 | 150 | 78.34 ± 0.019 | 79.637 ± 0.036 | 96.531 ± 0.042 |
| 4 | 200 | 81.86 ± 0.060 | 84.932 ± 0.013 | 96.621 ± 0.002 |
| 5 | 250 | 86.00 ± 0.030 | 88.191 ± 0.095 | 96.703 ± 0.010 |
| 6 | 300 | 91.04 ± 0.014 | 93.258 ± 0.154 | 96.945 ± 0.023 |
Table 4. Values of DPPH assay.
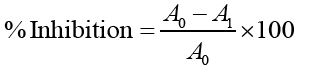
A0= Absorbance of Control, A1= Absorbance of the Standard/Extract.
Nanomedicine is an important field involving the use of various types of nanoparticles to treat cancer and cancerous cells. Synthesis of nanoparticles targeting biological pathways has become tremendously prominent due to the higher efficacy and fewer side effects of nanodrugs compared to other commercial cancer drugs. In this review, different medicinal plants and their active compounds, as well as green-synthesized metallic nanoparticles from medicinal plants, are discussed in relation to their anticancer activities. The use of plants in medicine is booming up. Now in the developed countries also people are returning to nature. Use of traditional medicine is the mainstay of primary healthcare, virtually in all developing countries. The reasons for the frequent use of traditional medicine being:
(i) the strong association of people with local flora and their belief on traditional knowledge regarding plants as medicine, So, this result clearly indicated that the particles are fairly stable due to the electrostatic repulsion. Three new oleanane-type triterpene saponins named grandibracteosides A-C.
ii) easy environment of the prepared nanoparticles. Previous studies have shown that the spherical Ag. NPs contribute to the absorption bands at around 425–475 nm in the UV-visible spectra. These absorption bands were assumed to correspond to the AgNP’s and Zn NP’s extra fine nature, with relatively small size (less than 15 nm). UV-Vis absorption spectra showed that the broad SPR band contained one peak at 420 and 340 nm for AgNP’s and ZnNP’s respectively. This peak illustrates the presence of homogeneous distribution of hydrosol Ag-NPs after 4 h of stirring times [15].
Zeta potential is an essential parameter for the characterization of stability in aqueous nanosuspensions. A minimum of ± 30 mV zeta potential values is required for indication of stable nanosuspension. At 4 h of stirring time, the zeta potential of AgNP’s and ZnNP’s was equal to -27.2 and -30.0 mV respectively were isolated from the methanolic extract of leaves of A. grandibracteata showed significant inhibitory activity against KB and MCF7 tumour cell lines in vitro. In this study, Methanolic extract showed the presence of phenolic compounds, the quantification of the phenolic content was carried out by using F-C reagent method as it contains mixture of phosphomolybdate and phoshotungstate which reacts with the phenolic content present in the drug in the presence of alkaline medium by the transfer of electrons. This is detected by the formation of the blue chromophore due to phosphomolybdate and phoshotungstate complex, considering the absorbance at 765 nm. Then, All, MEAL, AL AgNP’s and Al ZnNP’s were subjected to in-vitro antioxidant activity, in which all three sample showed significant result but Al AgNP’s shows better activity compared to other.by reducing DPPH stable free radical by donating hydrogen electron which leads to the formation of DPPH-H.