ISSN: 2347-7830
ISSN: 2347-7830
1PG Department of Zoology JP University Chapra, Bihar, India
2Department of Zoology, SMD College MN Jalalpur, Gopalganj, Bihar, India.
3Department of Botany, SMD College MN Jalalpur, Gopalganj, Bihar, India
Received date: 05 May 2013 Accepted date: 03 July 2013
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
The groundwater plays crucial role as a decentralized source of drinking water for millions cannot be overstated. Groundwater is generally less susceptible to contamination in comparison of surface water bodies. Also, the natural defect in rainwater gets removed while infiltrating through different soil levels. But, In India, the groundwater is used for irrigation and industrial work, a variety of land and water based activities are causing pollution of this resource. Its over-exploitation is causing aquifer contamination, while insufficient knowledge of groundwater flow dynamic and geo-hydro chemical process has led to its mineralization. The pump and treat remediation is used generally in case of contamination by hazardous wastes. This technique filters groundwater as a hydro chemical process through flow in columns with pores to filter molecules. We observed role of Absorption/desorption and dissolution of non-aqueous phase liquids on the effectiveness. The results showed that the remediation of groundwater depends directly on the physical/chemical properties of the contaminants and the hydrogeology of the site. The remediation of groundwater contaminated by hazardous waste using P and T technique is limited and expensive for mass pollution.
Contamination, Hydro chemical Process, Groundwater, Pump and Treat, Hazardous Waste, Non- aqueous Phase Liquids.
Groundwater contamination may be divisible into point (through salinity, fluoride and arsenic) and non-point (through fertilizers and Pesticides) pollution. Intensive use of Chemical fertilizers for agriculture and disposal of waste on land result in leaching of the residual nitrate causing high concentration in groundwater. Pollution of groundwater due to industrial efficient and municipal waste in water bodies is another major concern in many cities and industrial clusters in India. There are no estimates on the public health through groundwater pollution due to complexity in methodology and logistic problems. However, levels of toxicity depend on the type of pollutant. Mercury causes impairment of brain functions and disruption of the endocrine systems. High fluoride in water is often detected from damaged joints and bone deformities with increase in frequency of sister chromatic effect indicating its geotaxis effect. Arsenic contamination causes arsenicosis, for which there is no effective treatment.
Groundwater contaminated with dissolved organic and inorganic materials can be pumped out of the aquifer and be treated on the surface of the ground. The treated groundwater can either be returned to the pumped aquifer or used, or discharged. Pump and treat systems are designed with hydraulic to control the movement of contaminated water and reduces dissolved pollutant to restore the aquifer. The pumped groundwater with biological treatment, activated carbon absorption, air stripping of volatiles and metal precipitation become most effective for normal use of water. The pump and treat remediation requires the passage of groundwater through the section of the aquifer to remove dissolved pollutants and ones that desorbs from the geologic media with hydraulic conductivity. Pumping at higher rates may be limited by low permeability of sections in aquifer or inter phase mass transfer. The remediation time may be approximated by the composition of the contaminant rate of removal, the initial mass and the mass remaining in the aquifer in the aquifer at any point in time. In practice, Pump and treat system to drinking water standards is effective, perhaps only for areas with simple geology but difficult in area with complex geogenic activities. The pump and treat system to monitor contaminant concentration in the time reveals "Tailing" and "rebound" concept. "Tailing" refers to the slower rate of dissolved contaminant concentration decline with continued operation. "Rebound" refers appearance of dissolved contaminant concentrations in case of discontinuation in pumping. These both concept may result from contaminant description, Non-aqueous phase liquid (NAPL) dissolution, Precipitate dissolution, Groundwater velocity variation and matrix diffusion.
We examined water for its status as drinking water and estimated efficiency of Pump and treat method with application samples through experiments and mathematical simulations. We applied water contaminated with pesticide (Bromacil, Lindane and DDT) and petroleum products. For pesticide, we computed the expected remediation time for a contaminated section 10 m long, 100 m wide and 2 m thick. This section was located close to the source and contains the highest dissolved pesticide concentration uniformly distributed. In order to assess the effect of NAPL pool presence on the effectiveness of pump and treat remediation, we were considered the removal of toluene pools floating at the water table, by dissolving into groundwater. The original lengths of the pools were 100, 500 and 1000 cm. The thickness of all pools was considered to be uniform and equal to 1% of the respective length.
In general, the dissolved contaminants move slower than groundwater due to absorption in aquifer depends upon, contaminant properties and characteristics of the geologic material. The high level of contaminant move slowly in groundwater and dissociation is difficult resulting less efficient removal by pump and treat method. There tailing effect results in significant increase of remediation time and of the volume of groundwater. Also, if pumping of groundwater is ceased before the total contaminant quantity is removed from the aquifer, the concentration in groundwater might be significant increased due to release by slow desorption. The P and T remediation is accomplished by employing a series of wells located on a straight line and covering the entire width of the contaminated section. This well arrangement secures a one dimensional uniform flow in the contaminated section of the aquifer. The concentration in the wells can be simulated with the one dimensional advection desorption – absorption equation as :
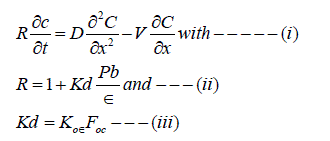
Where: C = Contaminant concentration (ML-3),
t = time,
x = longitudinal Cartesian coordinate (L),
V=average pore velocity in the x direction ( L .t -1),
R= retardation factor
D = Hydrodynamic dispersion coefficient in the x direction (L2t-1),
Kd = Linear absorption coefficient (L3.M-1)
Pb = Bulk density of the geologic medium (ML-3)
ε= porosity of the geologic medium,
Koε = linear absorption coefficient normalized for organic carbon content (L3.M-1),
Foc = fraction of organic carbon.
The fused retardation factors in the simulations are 1.5 for bromacil, 52 for lindane and 8330 for DDT. The value of lindane and DDT were computed based on the respective linear absorption coefficient valves and the organic carbon fraction of the aquifer, Foc = 0.009 using equation (2) and (3) [4]. The vale for bromacil was calculated through experiment by computing elution value of NaCl added to the top of the interested soil column.
The hydrodynamic dispersion coefficient was determined using:-
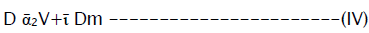
Where α2 = longitudinal dispersivity (L),![]() medium tortuosity and Dm = molecular diffusion coefficient (L2t-1)
medium tortuosity and Dm = molecular diffusion coefficient (L2t-1)
Estimation of the  were obtained from Gelhar et al [2]. The average pore velocity of groundwater used in the simulation was 50 m yr-1 (13.7 cm d-1) The diffusion component of the hydrodynamic dispersion coefficient was ignored due to the relatively high groundwater velocity during the remediation phase. According to equation (II) and use of input parameters for initial and final conditions, we simulated remediation by pump-and-treat method through aquifer section (fig. 2).
were obtained from Gelhar et al [2]. The average pore velocity of groundwater used in the simulation was 50 m yr-1 (13.7 cm d-1) The diffusion component of the hydrodynamic dispersion coefficient was ignored due to the relatively high groundwater velocity during the remediation phase. According to equation (II) and use of input parameters for initial and final conditions, we simulated remediation by pump-and-treat method through aquifer section (fig. 2).
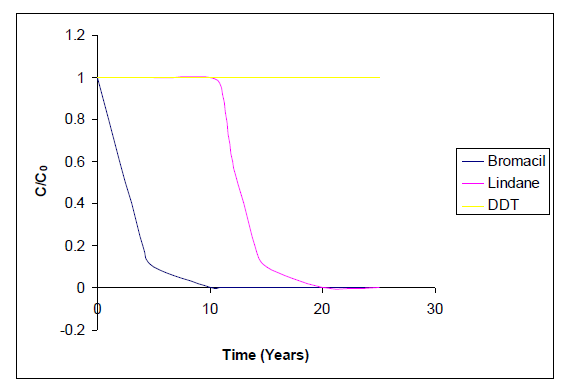
The maximum contaminant level was maintained 5% of detected level. The remediation time for each pesticide follows the order of the respective retardation factor as 1, 14 and unlimited years for bromacil, lindane and DDT respectively. The pore volume detected 5 and 70 for wells of 100m and 2 m thickness of aquifer section in respect of bromacil and lindane. Remediation of the entire length of well would require a much larger volume to be pumped and treated above ground. The value of R and accordingly the aquifer remediation time would increased with respective increase in organic carbon fraction.
Non-aqueous phase liquids (NAPLs) are liquids immiscible with water. When a NAPL is released, its portion will become immobilized and the remaining fluid will continue to move downward. The movement continues, until they either encounter an impermeable zone or spread out to the point where the pressure head is depleted and the NAPL forms residual. The NAPL groundwater may become unstable and manifested by "fingers" of NAPL penetrating the aquifer [6]. When fingers reach an impermeable zone, they can move laterally and follow the slope of the barrier. Thus, pools of NAPLs at near saturation mile form on layers of lower permeability media within an aquifer, or on the bottom of an aquifer, after displacing most of the pore water [5]. NAPLs lighter than water (e.g. petroleum products) tend to spread laterally and form a pool floating at the capillary fringe, above the water table [7]. The water table also moves up and down in response to seasonal recharge discharge and local pumping. When pools occur in the subsurface, NAPL dissolving in the water being transported to a well at concentrations exceeding drinking water standards and results in groundwater contamination. The NAPL solubility is higher than the maximum contaminant level for drinking water. Therefore, a small amount of NAPL contaminate a large volume of groundwater may last for many years [7].
Voudrias and Yeh [7] proposed that an extremely large number of pore volumes was required. Mass balance calculations should that removal of pure to were by 98.6% and benzene by 94.6% by dissolution in to groundwater required 132 and 53 pore volumes of groundwater. The required amount of groundwater for blob dissolution is given by following equations simulated by Borden and Piwani [1] as :
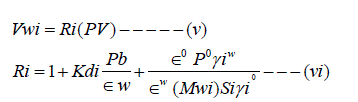
Where Vwi = Volume of water to flush compound (L3);
PV = aquifer pore volume (L3),
Ri = Linear equilibrium retardation factor for compound i due to partition in the solid phase and oil;
Pb = Linear absorption coefficient of Compound i (L3M-1)
Wio= Superscripts for water and oil (NAPL) phase;
ε = Porosity for water or oil phase,
γi = Activity coefficient of compound i for water or oil phase;
Po = density of oil phase (ML-3);
MWi = Molecular weight of compound i ;
Si = aqueous solubility of component i in its pore form (ML-3).
The above equation is limited for equilibrium of organic liquids in water. The time to complete dissolution will be :-
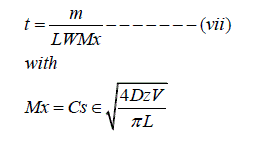
and, m = LWHoε
Where t = pool dissolution time (t), m = mass of NAPL in the pool (M), L = length of the pool, W = width of the pool,
H = Height of the pool, Mx = Mass transfer coefficient
(ML-2 t-1), Cs = NAPL solubility in water (ML-3) and ? = aquifer porosity.
The expression for the mass transfer coefficient is given by Johnson and Pinkow [3]. Using equation (7), the respective removal time of the pools was computed.
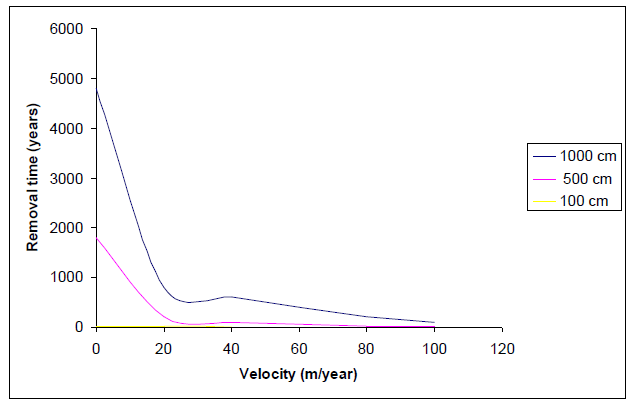
According to figure (3), the removal time for groundwater velocities < 20 m yr-1 renders the efficiency pool removal time decreased with solubility increase of the respective NAPL : Toluene < 1, 1, 2 – Trichloroethylene (TCE) < Benzene < 1, 1, 2 – Trichloroethane (TCA).
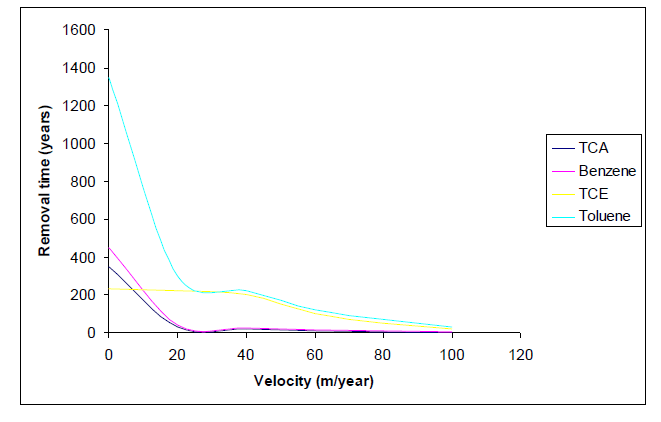
For complete NAPL removal, the desorption time of the dissolved contaminants, due to the tailing effects.