ISSN: 2347-7830
ISSN: 2347-7830
Department of Environmental Science, Bangalore University, Bangalore 560056, India.
Received date: 01/5/2015; Accepted date: 23/6/2015; Published date: 29/6/2015
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
Ca-Mg-HCO3 is the major hydrochemical facies in the study area, with cation facies belonging to Ca-Na (95.84%) and Ca-Mg (4.16%) and the anion facies being Cl-SO4-HCO3 (100%). The concentration of alkaline earths exceeded that of alkalies (viz., Ca+Mg > Na+K) and concentration of weak acids exceeded that of strong acid elements (viz., HCO3 > Cl + SO4). Indirect base-exchange reaction was noticed in 95.8% of the samples, which involved ion exchange of Ca2+–Mg2+ in water with Na+–K+ in rocks. Permanent/noncarbonate hardness was noticed in 95.84% of the samples due to higher total hardness values over total alkalinity. Radon activity in 87.5 % of the samples was higher than the EPA advised MCL value of 11.1 Bq/L. Due to higher water quality index (58.0 > WQI < to 137.8) in majority of the water samples, overall groundwater quality in the study area is categorized as unfit for human consumption, attributed to higher salinity, dissolved solids, hardness, etc. The groundwater samples are considered safe for irrigation purpose based on %Na and SAR values.
Bangalore north taluk, Piper, Radon, Chloroalkaline indices, Total cations, Total anions
As a resource, supply of groundwater is gaining increasing importance in the areas where surface waters are very scarce or absent and understanding characteristics is crucial for groundwater management [1]. Being a dynamic resource, groundwater is undergoing modifications both quantitatively (with a variety of pollutants generated from diverse sources (agricultural, industrial and domestic) and qualitatively. Although, the occurrence of groundwater is mainly associated with fracture and joint systems in an aquifer, evaluating groundwater quality in an area in terms of its chemical composition favors in gathering data on the environments through which the water is circulated by demonstrating key controlling factors. This can be used as a measure of its suitability for human and animal consumption, irrigation, and for industrial and other purposes besides their pollution status. Consequently, the groundwater quality is influenced by various natural processes like variation in climatic conditions, geological structure, composition of precipitation, residence time of water, aquifer materials and their interactions, and inputs from soil during percolation of water [2-4] besides intermixing among the different groundwater reservoirs along flow path in the subsurface. Anthropogenic inputs like concentrated sources of pollution such as industrial discharges, landfills and subsurface injection of chemicals and hazardous wastes are also considered as an obvious source of groundwater pollution [5]. Alternately, geochemical compositions of groundwater are regulated by several hydrogeochemical processes that the groundwater undergoes over space and time such as weathering of carbonates, evaporate and silicate minerals as well as ion exchange progressions [6,7]. The interaction of these factors result in leaching of surfacial salts to the water [8], resulting in the formation of various hydrochemical facies [9] and thus responsible for the seasonal and spatial variations in groundwater quality.
In the fast growing city like Bangalore, the quality of water is getting vastly deteriorated due to increasing human activities, unscientific waste disposal, improper water management and carelessness towards environment, leading to scarcity of potable water affecting the human health. Hence, an attempt was made in the present study to examine the controlling factors of hydrogeochemical processes by using various graphical methods and different hydrogeochemical ratios besides categorization of groundwater samples for utilitarian purposes. Further, radiation dose to public from ingestion of water-borne radon and inhalation of indoor radon are thought to be a higher threat than all other contaminants in water due to its carcinogenic effect. This is because radon is considered as the second leading cause of lung cancer next to smoking [10] contributing to almost 50% of the worldwide mean effective dose to the community [26] from natural means. Domestic water with elevated level of radon can make major contribution to the indoor radon exposure [11]. Hence, the present study is a pilot scale study that was carried out to calculate effective dose due to inhalation and ingestion of water-borne radon in order to demonstrate that the radiation dose from radon in drinking water is on average low relative to that from the inhalation of radon present in indoor air.
Location and Extent of the Study Area
Bangalore district is situated in the heart of the South-Deccan plateau in peninsular India to the South-Eastern corner of Karnataka State between the latitudinal parallels of 12° 39' N & 13° 18' N and longitudinal meridians of 77° 22' E & 77°52'E at an average elevation of about 900 meters covering an area of about 2,191 sq.kms (Bangalore rural and urban districts). Among the four taluks (viz., Anekal, Bangalore North, South and East), Bangalore North taluk is having more or less a level plateau / flat topology with major portion lies between 839 to 962 m above MSL. The taluk is characterized by various geological formations belonging mainly to the Archean and Upper Proteozoic periods (Figure 1). Granites and Gneisses of peninsular gneissic group constitute major aquifers in the urban district of Bangalore. Laterites of Tertiary age occur as isolated patches capping crystalline rocks in Bangalore north taluk. Peninsular Gneisses rock groups being metamorphic rocks cover major portion of the taluk followed by small patches of Closepet granite and sargur/satyamangalam rock groups towards the south and north-west respectively. Ground water occurs in phreatic conditions or unconfined conditions in the weathered zone and under semi confined to confined conditions in fractured and jointed rock formations. The occurrence of ground water movement and recharge to aquifers are controlled by various factors like fracture pattern, degree of weathering, geomorphological setup and amount of rainfall received. Red loamy and sandy soils occur on hilly to undulating land slope on granite and gneissic terrain in the eastern and southern parts of Bangalore north while laterite soils can be seen in western parts of Bangalore North. The drainage pattern of the Bangalore North taluk is governed by the Granitic ridge running NNE to SSE almost to the middle of the taluk. Bangalore has no major rivers flowing in the district. The Arkavati River flows in the district for a small distance in Bangalore North taluk and the Dakshina Pinakini touches the borders of the district to the North-East of the Anekal taluk. The climate is classed as the seasonally dry tropical savanna climate with four seasons. The dry season with clear bright weather is from December to February with summer from March to May (Characterized by high temperatures), followed by the southwest monsoon season from June to September. October and November constitute the post-monsoon or retreating monsoon season. The main features of the climate of Bangalore are the agreeable range of temperatures, from the highest maximum of 330C in April to the lowest minimum of 140C in January. The mean monthly relative humidity is the lowest during the month of March at 44% and records highest between the months of June and October at 80 to 85%. The mean annual rainfall is 859.6 mm, with two rainy seasons, June to September and October to November, come one after the other but with opposite wind regimes, corresponding to the southwest (S-W) and northeast (N-E) monsoons; 54% of the total annual rainfall is being received during the S-W monsoon period.
A total of 24 groundwater samples (viz., boreholes) were collected from Bangalore north taluk in a 1 L pre-cleaned polyethylene bottles during pre-monsoon season of April 2012. The samples were taken at least 10 min after removal of water from the tube well/borehole by pumping in order to get a representative sample that has not been in the pipe for an extended period of time. The groundwater quality inventory survey involved comprehensive physico-chemical attributes like pH, electrical conductivity (EC), total dissolved solids (TDS), total alkalinity, total hardness (TH), dissolved oxygen (DO), and major ions (Ca2+, Mg2+, Na+, K+, Fe2+,F-, Cl-, HCO3–, SO42-, NO3–, PO43-). All the parameters were analyzed by employing APHA [12] recommended standard analytical procedures. Fifteen water quality parameters were considered for WQI calculation using equation given in Table 2.
Radon analysis and effective dose calculation
Radon concentration in the ground water samples was measured using RAD7 radon analyzer (Durridge Co., USA) with RAD H2O accessory attached employing closed loop aeration concept. Detailed information on the radon activity measurement techniques is given elsewhere [13,14]. The annual mean effective doses from ingestion and inhalation of water-borne radon were calculated using the parameters established in the UNSCEAR [26] report which is as follows:
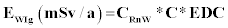
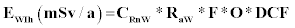
Where EWIg is the effective dose for ingestion, EWIh is the effective dose for inhalation, CRnW is the radon concentration in water (kBq/m3 or Bq/L), CW is the weighted estimate of water consumption (60 L/a) and EDC is the effective dose coefficient for ingestion (3.5 nSv/Bq) respectively, RaW is the ratio of radon in air to the radon in water (10-4), F is the equilibrium factor between radon and its progenies (0.4), O is the average indoor occupancy time per individual (7,000 h/a) and DCF is the dose conversion factor for radon exposure [9 nSv/(Bq h/m3)].
Tables 1 and 2 summarizes results of physico-chemical and irrigational quality parameters, water quality index, chloroalkaline indices, radon activity and the resultant effective dose.
pH of the groundwater samples from Bangalore north taluk varied from 6.4 to 9.8, majority of the samples falling in alkaline category. It was found that 4.17% and 41.67% of the samples respectively showed pH value below and above BIS standard limit of 6.5-8.5 (Table 1). The temperature of the samples was in the range of 27.5 to 31.2°C. The electrical conductivity and total dissolved solids respectively ranged from 355 to 1345 μS/cm and 220 to 834 mg/L. Conductivity was below the BIS standard of 2000 μS/cm [15] in all the samples while 62.5% samples showed higher total dissolved solids above the BIS desirable limit of 500 mg/L [16]. The total hardness value varied from 402.4 to 814.8 mg/L and all the groundwater samples are categorized as very hard water (viz., TH> 300 mg/L)[17]. It was also found that 70.83% of samples had their hardness value above the BIS desirable limit of 300 mg/L and 29.17% of the samples above the BIS permissible limit of 600 mg/L. Total Alkalinity of the water samples ranging from 316 to 524 mg/L was under permissible limit of 600 mg/L (Table 1). Also, 95.84 % of groundwater samples showed higher total hardness over total alkalinity values indicating that water is characterized by non-carbonated / permanent hardness. Calcium hardness value ranged from 312.5 to 507.5 mg/L, with a mean value of 389.9 mg/L.
Among the alkaline earth metals, the concentration of calcium and magnesium ranged from 125.0 to 203 mg/L and 15.5 to 82.3 mg/L respectively. It was found that 91.66% of samples had calcium values above the desirable limit of 75 mg/L and 8.34% samples above the permissible limit of 200 mg/L. In contrast, 58.34% of samples showed magnesium concentration below the limit of 30 mg/L and 41.66% samples below the permissible limit of 100 mg/L. Among alkalies, sodium concentration ranged from 50.9 to 96.3 mg/L and potassium concentration varying from 20.1 to 106.6 mg/L. It was observed that sodium content was well below the desirable limit of 100 mg/L but, potassium was above the limit of 10 mg/L in all the samples. Ferrous iron concentration ranged from 0.13 to 0.41 mg/L and it was found that the iron content was above the desirable limit of 0.3 mg/L in 45.84% of samples (Table 1).
Among anions, the bicarbonate was the dominant ion, with its concentration in the range of 385.5 to 513.6 mg/L. Chloride content ranging from 26.5 to 151.9 mg/L was well below the desirable limit of 250 mg/L. Sulphate concentration ranging from 19.5 to 54.2 mg/L was well below the desirable limit of 200 mg/L. Fluoride concentration varied from 0.2 to 1.3 mg/L and was above the desirable limit of 1.0 mg/L in 8.34% of the samples. Phosphate concentration ranging from 0.1 to 2.2 mg/L was above the limit of 0.3 mg/L in 91.66% of samples. Nitrate content was in the range of 10.5 to 47.0 mg/L and only 12.5% of the samples showed higher nitrate content above the limit of 45 mg/L.
Hydrochemical Facies
Piper trilinear diagram [18] was plotted using meq/L concentration of major cations (Ca2+,Mg2+, Na+, K+) and anions (HCO3-,SO42- and Cl-) revealed that major water type prevailed in the study area was Ca-Mg-HCO3 with calcium (Ca2+) and bicarbonate (HCO3-) being the dominant cation and anion. It is also very distinct from the Figure 2 that the alkaline earths exceeded the alkalies concentration (viz., Ca+Mg>Na+K) and weak acids exceeded the strong acid elements concentration (viz., HCO3>Cl + SO4). Further, major cation facies was Ca-Na (95.84 %) followed by Ca-Mg (4.16%) while the major anion facies was found to be Cl-SO4-HCO3 (100%). In the present study, Chloroalkaline indices 1 and 2 [19] values varied from (-5.365) to 0.325 and (-0.518) to 0.119 respectively (Table 2), with 95.84% of the samples showing negative values for both indices. This reflect the dominance of indirect base exchange reaction (viz., chloro-alkaline disequilibrium or reverse ion exchange) involving cation exchange of Na+ and K+ in the aquifer substrate against Mg2+ and Ca2+ in the groundwater [20,21].
The ratio of (Ca2+ + Mg2+) / T-cations being greater than 0.5 (Table 3) clearly showed the contribution of alkaline earth metals to total cationic concentrations. This fact is further supported by the low (Na+ + K+) / T-cations ratio, being less than 0.5 (Table 3). The (Ca2+ + Mg2+) versus total cations plot (Figure 3) shows that the concentration of (Ca2+ + Mg2+) mostly falls near 1:1 line, indicating contribution of the carbonate weathering being the major source of dissolved ions [21]. Further, low (Na+ + K+) / T-cations ruled out the contribution of cations via silicate weathering. The scatter plot of (Na+ + K+) versus total cations (Figure 3) shows that all samples fall below 1:1 line with low equivalent ratio, which indicates that there is very small contribution from silicate weathering [22]. Further, the effects of ion exchange was evaluated using Na+ / (Na++Cl-) diagram against total dissolved solids [23], in which majority of the samples fall in the ion exchange field (Figure 4).
The ratio of (HCO3- + SO42-) / T-anions ranged from 0.589 – 0.895 (Table 3) and this value being less than 1 depicted the effect of the rain on the water/rock interaction with deficit of HCO3-and SO42- [24].Otherwise, excess bicarbonate and sulfate ionic concentration could have been noticed in the groundwater samples. Further, Cl-/T-anions ratio ranged from 0.076 – 0.361 (Table 3) and is less than 1 in all the groundwater samples. But, higher ionic ratio of (HCO3-+SO42-) / T-anions over the ratio of Cl-/T-anions (Figure 3) rule out the fact that lack of rain and the decomposition of organic matter by bacterial organisms in the soil would provide the appropriate CO2to the rock-water interaction [24]. All the groundwater samples showed Cl−/HCO3- ratios lower than 0.5 illustrating that the groundwater was unaffected or freshwater and hence not affected by salinization.
Suitability of groundwater
Water quality index ranged from 58.0 to 137.8 (Table 2) illustrating that all the samples were either moderate to severely pollute. It can be inferred that none of the samples fall under excellent to good category. Among 24 groundwater samples, 20.83% of the samples, each falling under poor (58.0>WQI<74.1) and very poor (76.3>WQI<93.7) category can only be used for drinking after conventional treatment and disinfection. Further, water quality at 58.34% of the sampling stations (101.3>WQI<137.8) are categorized as unfit for consumption (Table 4), which could be used for aquaculture, industrial and irrigation purposes. Alternately, the groundwater samples can be suitably used for irrigation purpose as they belong to safe and excellent category (Table 4) based on percent sodium (14.7>% Na<27.1) and SAR (0.94>SAR<1.88) values (Table 2).
Correlation Matrix
Correlation coefficient is commonly used to establish the relationship between independent and dependent variables. The correlation matrix of 17 parameters, for the 24 samples in the study area is given in Table 5.
The high correlation between EC and TDS reflects the interdependency of these measurements as general measures of the amount of total dissolved solutes. The correlation between EC and TDS (r=1.00) is due to the fact that conductivity depends on total dissolved solids. There existed positive correlation between EC and dissolved ions in water as revealed by the relation between EC and Fe (r=0.70), EC and Cl (r=0.65), EC-Ca (r=0.57), EC-PO4 (r=0.51), EC-NO3 (r=0.45), EC-Mg (r=0.44), EC-HCO3 (r=0.22) and EC-SO4 (r=0.21). Total dissolved solids showed similar trend by displaying positive relation with Fe (r=0.70), Ca (r=0.56), Mg (r=0.44), Cl (r=0.66), HCO3 (r=0.24), SO4 (r=0.25), NO3 (r=0.47) and PO4 (r=0.51).
The fact that hardness in water is caused primarily by the presence of cations such as calcium and magnesium and anions such as carbonate, bicarbonate, chloride, and sulfate is supported by positive and strong relationship between TH and CaH (r=0.83), TH and Ca (r=0.83) and TH and Mg (r=0.84), TH and HCO3 (r=0.30), TH and Cl (r=0.42) and TH and SO4 (r=0.19), Ca and Cl (r=0.36), Ca2+-HCO3- (r=0.30), Mg and SO4 (r=0.33), Mg and HCO3 (r=0.21), Mg and Cl (r=0.34) and Ca and Mg (r=0.39). The correlation between TA and HCO3 (r=1.00) and TA and Ca (r=0.30) and TA and Mg (r=0.21) is due to the fact that total alkalinity is caused primarily due to the presence of carbonates and bicarbonates in water.
Influence of saline water was ruled out because of very low, but positive correlation between Na- Cl (r=0.09). The relation between Ca-HCO3 (r=0.30) and Ca-Na (r=0.30) may represent contributions from silicate and carbonate weathering while the positive correlation between Mg-SO4 (r=0.33) may represent gypsum dissolution. The correlation between K-NO3 (r=0.23) may represent poor sanitation conditions including application of fertilizer.
The positive correlation was also observed between Fe and Cl (r=0.79), Fe and NO3 (r=0.46), Fe and PO4 (r=0.49), Fe and HCO3 (r=0.30), K and NO3 (r=0.23), Na and NO3 (r=0.32), Mg and NO3 (r=0.41), Na and K (r=0.21), Mg and PO4 (r=0.27), Ca and NO3 (r=0.53), Ca and PO4 (r=0.15), Cl and HCO3 (r=0.21), Cl and Na (r=0.09), Na and HCO3- (r=0.04), TA and Cl (r=0.21), TA and NO3 (r=0.28), TA and PO4 (r=0.39), TA and F (r=0.24), TA and SO4 (r=0.13), TA and Fe (r=0.30), Na and TDS (r=0.04) and Na and EC (r=0.07). Although many of the above relations indicate that they most likely derive from the same source of water, some relations were found to be insignificant due to low correlation coefficients. Further, the increase of one element will or might have increased the concentration of the other and synergistic behavior amongst the dissolved ions in water or even might have derived from anthropogenic sources besides natural sources.
Weathering of HCO3, Ca, Mg, Na, K, H4SiO4 and leaching of ions like Cl, SO4 enhances the Uranium concentration in groundwater [25], which in turn is responsible for elevated concentration of radon. This fact hold good to the present study to some extent due to establishment of positive correlation between Rn and temperature (r=0.33), Rn and K (r=0.18), Rn and F (r=0.16) and Rn and SO4 (r=0.06) illustrating that radon flux from rocks / soil to groundwater depends upon temperature besides impact of weathering and leaching processes. Correlation between radon and other physico-chemical parameters was less significant as the correlation coefficient was moderate to highly negative.
Radon Activity and Mean Annual Effective Doses
The radon activity in groundwater samples ranged from 1.5 to 381.2 Bq/L (Table 2), with 87.5% of the samples showing radon activity above the EPA (1999) proposed maximum contaminant level (MCL) of 11.1 Bq/L L (300 pCi/L or 11.1 kBq/m3). Further, the mean annual effective doses for ingestion (stomach), inhalation (lungs) and whole body (ingestion + inhalation) from drinking water were computed to be 0.017 ± 0.022 (range: 0.0003 to 0.0805), 0.205 ± 0.265 (range: 0.004 to 0.961) and 0.222 ± 0.287 (range: 0.004 to 1.041) mSv/a respectively. These mean values are well below the WHO reference level of 0.1 mSv/a of WHO and hence do not pose any health problem. Further, the mean annual effective doses due to ingestion (0.017 mSv/a) and inhalation (0.205 mSv/a) of water-borne radon in the present study are higher than the UNSCEAR [26] proposed mean annual effective doses due to ingestion (0.002 mSv/a) and inhalation (0.025 mSv/a).
Hydrochemical facies in the study area belong to Ca-Mg-HCO3, with cation facies belonging to Ca-Na (95.84%) and Ca-Mg (4.16%); while the anion facies was found to be Cl-SO4-HCO3 (100%). The alkaline earths exceeded the alkalies concentration (viz., Ca+Mg>Na+K) and weak acids exceeded the strong acid elements concentration (viz., HCO3>Cl+SO4). The groundwater samples are categorized as very hard with 95.84% of the samples showing permanent or non-carbonate hardness. Due to higher water quality index in majority of the water samples, overall groundwater quality in the study area is categorized as unfit for human consumption, attributed to higher salinity, dissolved solids, hardness, etc. But, the groundwater samples are considered safe for irrigation purpose based on %Na and SAR values. Negative chloroalkaline indices in 95.8% of the samples revealed that the groundwater chemistry is influenced by water–rock interaction and aquifer material mineralogy (viz., ion exchange between Ca2+–Mg2+ in water and Na+–K+ in rocks). There existed positive correlation between EC and other dissolved ions, revealing they most likely derive from the same source of water, although some inter-ionic relations were found to be insignificant due to low correlation coefficients. Further, the radiation dose from radon in drinking water is on average low relative to that from the inhalation of water-borne radon present in indoor air. Proper mitigation measures like increasing indoor ventilation by means of open windows, air-to-air heat exchangers, filters, fans, etc., should be promoted for removal of radon from indoor air.