e-ISSN: 2347-7857 p-ISSN: 2347-7849
e-ISSN: 2347-7857 p-ISSN: 2347-7849
Adenike Okunlola*, Aishat Kehinde Lawal
Department of Pharmaceutics and Industrial Pharmacy, University of Ibadan, Ibadan, Nigeria
Received: 10-Jul-2020, Manuscript No. JPN-20- 003-PreQc-22; Editor assigned: 14-Jul-2020, Pre QC No. JPN-20- 003-PreQc-22(PQ); Reviewed: 24-Jul-2020, QC No. JPN- 20- 003-PreQc-22; Revised: 27-Jul-2022, Manuscript No. JPN-22- 003-PreQc-22(R); Published: 12-Sep-2022, DOI:10.4172/23477857.10.4.003.
Visit for more related articles at Research & Reviews: Journal of Pharmaceutics and Nanotechnology
Bilayer tablets can control the delivery rate of either a single or two different active pharmaceutical ingredients, solving the problem of repetitive dosing associated with conventional tablets. The use of suitable excipients such as modified starches can control the release of drugs from the bilayer tablets while imparting mechanical strength. The aim of the research is to formulate bilayer tablets of metformin hydrochloride using bitter yam starches (Dioscorea dumetorum) modified by Carboxymethylation and acid hydrolysis as immediate and sustained release polymers respectively. The native and modified starches were characterized using morphology, FTIR, density and flow properties. Bilayer tablets of metformin hydrochloride were prepared using carboxymethylated (2.5 and 5% w/w) and acid-modified (10.0% w/w) bitter yam starches with Sodium Starch Glycolate (SSG) and Hydroxy Propyl Methyl Cellulose (HPMC) as standards. The bilayer tablets were evaluated for Crushing Strength (CS), Friability (FR), Disintegration Time (DT) and dissolution time, t90 (time taken for 90% drug release). The degree of substitution of carboxymethylated bitter yam starch was 0.38. The swelling of the starch increased with carboxymethylation but reduced with acid hydrolysis. Modification of starch resulted in improved flow. Bilayer tablets containing the modified starches gave lower friability and higher CSFR/DT ratio than those containing SSG and HPMC. The bilayer tablets showed initial burst release to provide the loading dose of drug followed by controlled release up to over 10 hours. The in vitro dissolution kinetics generally followed the Higuchi and Korsmeyer-Peppas models.
Polymers; HPMC; carboxymethylation; Drugs
Bi-layer tablets are tablets that consist of two layers, in which one layer is formulated for immediate release of the drug with the aim of reaching a high serum concentration in a short period of time. While the second layer is for sustained release to maintain an effective plasma level for a prolonged period of time [1,2]. Drug release from fast releasing layer leads to a sudden rise in the blood concentration while the blood level is maintained at steady state as the drug is released from the sustaining layer [3]. Bilayer tablets are also suitable for sequential release of two drugs combined in a formulation as well as for separating two incompatible substances [4,5].
One of the major challenges in the formulation of bilayer tablets is lack of sufficient bonding and adhesion at the interface between the adjacent compacted layers which is often the result of an interfacial crack driven by residual stresses in the tablet propagating a finite distance within the tablet [6]. In addition, if the compacted layers are too soft or too hard, they will not bond securely with each other which can lead to compromised mechanical integrity. To produce a quality bilayer tablet that will meet validated and Good Manufacturing Practices (GMP) requirements, the tableting formulation and equipment should be capable of preventing capping and separation of the two individual layers that constitute the bi-layer tablet, providing sufficient tablet hardness, producing a clear visual separation between the two layers and preventing cross-contamination between the two layers [7]. Thus, in bilayer tablets, hardness and friability are two interrelated parameters that should be properly balanced such that the hardness is low enough as not to hinder the disintegration time while not compromising its friability. This implies that bilayer tablets should be strong enough to withstand handling after compression. The use of suitable excipients such as modified starches that can impart mechanical strength to the formulation of bilayer tablets is important in order to guarantee the mechanical integrity of these tablets.
Dioscorea dumetorum (bitter yam) is a climber crop that is common throughout the savannah region as it grows easily and is a heavy cropper. Native bitter yam starch has been utilized as a binder at concentration of 2.5%-10% w/w in chloroquine phosphate tablets in which it imparted high mechanical strength and prolonged drug release in comparison to the established corn starch BP [8]. In another study, Okunlola utilized carboxymethylated white yam starch and acid-modified bitter yam starch as immediate and sustained release polymers respectively in bilayer tablet formulations of aceclofenac [9]. In the modification of starch by carboxymethylation, introduction of large hydrophilic carboxymethyl groups disrupts the hydrogen bonding within the polymer structure [10]. This allows water to penetrate the molecule and the polymer becomes cold water soluble. It has been reported that the type of starch, particle size after chemical modification, degree of substitution and cross-linking, and the amount of soluble by-product of the reaction can affect the performance of the carboxymethylated starch. Carboxymethylated starch would provide fast disintegration in tablets and would therefore be useful in the fast release layer of bilayer tablets. Acid hydrolysis is an important chemical modification that can significantly change the structural and functional properties of starch without disrupting its granular morphology. Acid reacts and de-polymerizes the amorphous regions of the starch granules such that when heated beyond its gelatinization temperature, the granules rupture quickly. This result in a starch with lower viscosity which becomes a stronger gel on cooking compared to the native parent starch. The extent of modification depends on the acid concentration, reaction time and temperature. In a previous study, acid-modified tapioca starch was investigated as a filler- binder in direct compression. Tablets containing the acid-modified starch showed higher tensile strength, lower friability, and faster dissolution than the native tapioca starch. In another study, it was reported that spray-dried powder of acid-modified tapioca starch had good flow characteristic and higher compressibility when compared to some commercial fillers. Acid-hydrolyzed starch would therefore be suitable as a sustained release polymer.
Metformin hydrochloride is an oral anti-hyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. The drug has an oral bioavailability of 50%–60% under fasting conditions, peak plasma concentrations (Cmax) are reached within one to three hours of taking immediate-release metformin. The average elimination half-life in plasma is 6.2 hours thus making it a suitable drug as sustained release formulations. Thus, the aim of the research project is to formulate bilayer tablets of Metformin hydrochloride using carboxymethylated bitter yam starch and acetylated bitter yam starch as immediate and sustained release polymers respectively. The fast release of the drug is to ensure an immediate relief from hyperglycemia while the sustained layer ensures the maintenance of an effective plasma level. The mechanical and release properties of the bilayer tablets were evaluated in comparison to those containing superdisintegrant, Sodium Starch Glycolate (SSG) and the sustained release polymer Hydroxy Propyl Methyl Cellulose (HPMC) as standards.
The materials used were metformin hydrochloride (Zydus Cadila Healthcare Ltd., Ahmedabad, India); Purified Talc BP (Anmol Chemicals, Mumbai, India), Polyvinylpyrolidine (Zhengzhou Qiangjin Science and Technology Trading Co., Ltd. Henan, China) ; Isopropyl alcohol (BDH, England); Sodium Starch Glycolate (Sarchem Laboratories, New Jersey, USA); Hydroxy Propyl Methy Cellulose (Oxford Lab Chemicals, Maharashtra, India); Microcystalline cellulose (Schutz & Co., GmbH, Hamburg, Germany); Hydrochloric acid (BDH, England), sodium hydroxide (Sigma-Aldrich GmbH, Germany), Lactose (Meggle Excipients and Technology Wasserburg, Germany), monochloroacetic acid (Alfa Aesear, Massachusetts, USA). Bitter yam tubers were obtained from local farmers in Ibadan, Oyo State, Nigeria and authenticated (FHI no: 109674). All other reagents were of analytical grade.
Extraction of bitter yam starch
The tubers of bitter yam were washed with distilled water, peeled, washed again and then cut into small pieces. The pieces were milled into a fine paste using a laboratory mill and the slurry was strained through a muslin cloth. The filtrate was left to settle. The supernatant was decanted at 12 hour interval and the starch slurry re suspended in distilled water. The starch cake was collected, dried in a hot air oven at 50âÃâæC for 48 hours. The dried mass was pulverized using a laboratory blender and then screened through a 125 um mesh sieve.
Determination of the degree of substitution of carboxymethylated starch
Carboxymethylated starch (0.5 g) was dissolved in 20 mL of 0.2 M NAOH. Distilled water (50 mL) was then added and the resulting solution was transferred into a 100 mL volumetric flask and made up to volume with water. From this solution, 25 mL was transferred into a volumetric flask and diluted with 50 mL of distilled water. The excess NAOH was back titrated with 0.05 M HCL using phenolphthalein as indicator. The titration was done in triplicate and the mean value determined for the volume of HCL used. A blank titration was then carried out without the starch sample.
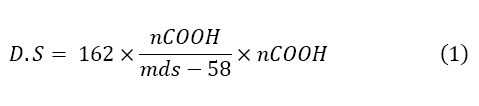
Evaluation of bilayer tablets of metformin hydrochloride
Bilayer tablets of metformin hydrochloride were prepared using dry granulation for the fast release layer (Formulations F1, F2, F3 and F4 containing carboxymethylated bitter yam starch and SSG) while the wet granulation method was used for the sustained release layer (Formulations S1 and S2 containing acid modified bitter yam starch and HPMC). The composition of formulations for the two layers is presented in Table 1.
| Ingredient (mg) | F1 | F2 | F3 | F4 | |
|---|---|---|---|---|---|
| Fast Release Layer | |||||
| Metformin hydrochloride | 250 | 250 | 250 | 250 | |
| Carboxymethylated bitter yam starch | 8.75 | 17.50 | - | - | |
| Sodium starch glycollate | - | - | 8.75 | 17.50 | |
| Aspartame | 7.0 | 7.0 | 7.0 | 7.0 | |
| Talc | 3.5 | 3.5 | 3.5 | 3.5 | |
| Magnesium stearate | 1.0 | 1.0 | 1.0 | 1.0 | |
| Microcrystalline cellulose (MCC) to | 350 | 350 | 350 | 350 | |
| Sustained Release Layer | |||||
| Ingredient (mg) | S1 | S2 | |||
| Metformin hydrochloride | 335 | 335 | |||
| Acid-modified bitter yam starch | 40 | - | |||
| HPMC | - | 40 | |||
| PVP | 10 | 10 | |||
| Talc | 4 | 4 | |||
| Sodium carboxymethyl cellulose | 11 | 11 | |||
Table 1. Formulations of the immediate and sustained release layers of the bilayer tablets of metformin.
Various combinations of the formulations for the fast and sustained release layers were used to prepare eight batches of bilayer tablets. The batches and the results of the tablet properties evaluated are presented (Table 1).
Weight uniformity and thickness
All the formulated batches of tablets passed the weight uniformity test as the permissible percentage deviation from the mean was ± 5% in conformity with the specifications of the United States Pharmacopoeia. The average values of thickness of the tablets were also found to be within acceptable limits of British Pharmacopoeia specifications.
Mechanical properties
Crushing strength evaluates the resistance of the tablet to chipping, abrasion or breakage under the conditions of storage, transportation and handling. Generally, a crushing strength of 40 N is normally considered to be the minimum for a satisfactory tablet even though there are no limits for its acceptance or rejection in tablet formulations, probably because the desired crushing strength is largely dependent on the intended use of the tablet. Crushing strength increased with an increase in concentration of polymer. The rank order of the tablet formulations was B1<B2<B5<B6<B7<B3<B4<B8. It was observed that tablet formulations containing HPMC had higher crushing strength than those containing the acid hydrolyzed bitter yam starch.
Friability test
Friability values provide a measure of tablet weakness. The friability tests for tablets is deigned to evaluate the ability of the tablets to withstand abrasion or packaging, transportation and general handling. By convention, tablets that lose not more than 1% of their weight after the friability test are considered acceptable. The rank order of the formulations was B1<B2<B3<B4<B5<B6>B7< B8. Generally, it was observed that friability values increased with increase in concentration of polymer. Tablets containing the modified bitter yam starch had lower friability than those containing standards. This implies that tablets containing bitter yam starches would be able to withstand abrasion during packaging, handling and shipping better than those tablets containing the standards.
Disintegration test
The disintegration time of a tablet is the time it takes for the tablet to completely disintegrate. Complete disintegration is defined as that state in which any residue of the unit, except fragments of insoluble coating or capsule shell, remaining on the screen of the test apparatus is a soft mass having no firm core. It was observed that immediate release layer in all the bilayer tablets disintegrated rapidly within a period of ≤ 5 minutes. However, the overall time for total disintegration was reported and presented. Some of the factors which could affect the disintegration time of tablets include nature of the polymers used, their concentration and applied compression pressure. The CSFR/DT ratio has been suggested as a better index of measuring tablet quality because in addition to measuring tablet strength (crushing) and weakness (friability), it simultaneously evaluates all negative effects of these parameters on disintegration time. In general, higher values of the CSFR/DT ratio indicate a better balance between binding and disintegration properties. The CSFR/DT ratios were highest for the combinations of carboxymethylated and acid hydrolyzed bitter yam starch as immediate and sustained release polymers. This means that tablets containing bitter yam starch gave better mechanical properties.
Dissolution testing is an in vitro method that characterizes how an active pharmaceutical ingredient is released from a solid dosage form into solution within the gastrointestinal tract. In vitro dissolution testing is considered to be reliable and rational in the prediction of in vivo drug bioavailability. It is an essential tool in the pharmaceutical analysis of dosage forms at various stages of formulation and development. The in-vitro dissolution of controlled release layer was studied for 12 hours and the time taken for 90% of drug release (t90) was determined. There was an initial sharp rise in the dissolution due to the fast release of metformin from the immediate release layer. This was then followed by a more steady and sustained release. Dissolution time increased with increase in concentration of the two polymers for all formulations. The formulation B4 containing acid modified starch with 5% w/w of SSG had at 90 value of >10.00 h, which was comparable to the dissolution time of formulations B5 and B6 containing HPMC with 2.5% w/w and 5% w/w of carboxymethylated bitter yam starch, respectively. Generally, tablets containing HPMC and acid-modified bitter yam starch with SSG as fast release layer, gave longer dissolution times than similar tablets containing carboxymethylated bitter yam starch as the fast release layer.
The physicochemical properties of drug and polymer have been shown to govern the release of drug from formulations which could affect their release kinetics. The kinetic models and the correlation coefficients obtained are presented (Table 2).
| Batch | Formulation | Zero order | First order | Higuchi | Hixson-Crowell | Korsmeyer | |
|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | n | ||
| B1 | F1S1 | 0.8605 | 0.8211 | 0.95 | 0.9068 | *0.9701 | 1.67 |
| B2 | F2S1 | 0.821 | 0.8687 | *0.9555 | 0.8856 | 0.9503 | 1.2 |
| B3 | F3S1 | 0.8364 | 0.8605 | 0.9335 | 0.9374 | *0.9402 | 0.95 |
| B4 | F4S1 | 0.821 | 0.8446 | 0.947 | 0.8557 | *0.9702 | 1.2 |
| B5 | F1S2 | 0.8211 | 0.873 | 0.947 | 0.9183 | *0.9702 | 1.01 |
| B6 | F2S2 | 0.873 | 0.8211 | 0.9714 | 0.8856 | *0.9804 | 0.9 |
| B7 | F3S2 | 0.8605 | 0.821 | 0.9399 | 0.9068 | *0.9901 | 0.87 |
| B8 | F4S2 | 0.8364 | 0.7384 | 0.947 | 0.8557 | *0.9904 | 0.82 |
Note: (*) Highest correlation coefficient for batch
Table 2. Correlation coefficients obtained for bilayer tablets of metformin using different kinetic models (n=3).
Drug release for the bilayer tablets generally fitted the Korsmeyer-Peppas model. In this model, the value of n characterizes the release mechanism of drug. When 0.45 ≤ n, this corresponds to a Fickian mechanism; 0.45<n<0.89 to non Fickian transport; n=0.89 to case II (relaxational transport) and n>0.89 to super case II transport. From the values of the n, the drug release mechanism from the formulations containing the modified bitter yam starches is generally considered to be super case II transport t, a non Fickian diffusion. Fickian and Non Fickian diffusion are two forms of diffusion that are described using Fick’s laws. Fickian diffusion obeys the Fickian laws whereas non Fickian diffusion does not obey the Fickian laws. The main difference between Fickian and Non Fickian Diffusion is the presence or absence of boundaries in the matrix system. Only Batch B2 containing acid-modified bitter yam starch and 5% w/w carboxymethylated bitter yam starch followed the Higuchi model of drug release. Higuchi describes drug release as a diffusion process that is square root dependent. Higuchi proposed this model of drug release based on the assumptions that the initial concentration of drug in the matrix is much higher than the drug solubility; matrix swelling and dissolution are negligible.
Modification of bitter yam starch by carboxymethylation produced starches with larger granules, improved flow and higher swelling power while modification by acid-hydrolysis resulted in larger size but reduced swelling power. The use of modified bitter yam starches as immediate and sustained release polymers in the formulation of bilayer tablets of metformin hydrochloride produced tablets with lower friability and higher CSFR/DT ratio, suggesting enhanced balance between mechanical strength and disintegration than those containing SSG and HPMC. The value of dissolution time was prolonged and the bilayer tablets generally fitted the Korsmeyer-Peppas release kinetics. Bitter yam (Dioscorea dumetorum) starch modified by carboxymethylation and acid-hydrolysis could be used as cheaper and more readily available alternative excipients in the formulation of bilayer tablet of drugs requiring high mechanical strength for immediate and sustained release.
[Crossref][Google scholar] [Pubmed]
[Crossref][Google scholar] [Pubmed]
[Crossref][Google scholar] [Pubmed]
[Crossref][Google scholar] [Pubmed]