e-ISSN: 2347-7857 p-ISSN: 2347-7849
e-ISSN: 2347-7857 p-ISSN: 2347-7849
Chandra Mohan SB1*, SB Puranik2, Prasanna Sagar3, Swamy Sreenivasa4, and Madhu Chakrapani Rao5
1Research Scholar, Bundelkhand University, Jhansi, Madhya Pradesh, India.
2East West College of Pharmacy, Bangalore, Karnataka, India.
3Gland Pharma Pvt Ltd, Hyderabad, Andhra Pradesh, India.
4Prof. C. N. Rao Center for Advanced Materials, Department of Chemistry, Tumkur University, Tumkur - 572 103, Karnataka, India
5Tadimety Aromatics Pvt. Ltd, Hirehally Industrial Area, Tumkur-572168, Karnataka, India
Received date: 18 January 2014 Accepted date: 14 February 2014
Visit for more related articles at Research & Reviews: Journal of Pharmaceutics and Nanotechnology
This article re-views the rationale for intellectual property protection in the development of new products useful for pharmaceuticals The foremost importance of the present article is it describes the new drug development process, stages involved in development, the importance of protecting the invention till approval of the new drug. The importance of patents to pharmaceutical innovation has been reported in several cross-industry studies by economists. Prior studies have found that inventions through patents play a more critical role in appropriating the benefits of innovation soma pills online in pharmaceuticals compared to other industries and a comparison of the Patent expiry of the blockbuster drug and its reduction in sales.
Patent, Pharmaceutical Industry, Formulation, Novelty, Blockbuster drug
A patent is a kind of Intellectual Property. The term patent can be defined as “a monopoly right conferred to the inventor who has invented a new product or process through his/her intellectual efforts capable of industrial application1,10. Patents are exclusive property rights in intangible creations of the human mind and it is awarded in recognition of innovation and more particularly the investment required to foster technical advance and the development of new ideas [1, 2].
The owner of a patent has the right to exclude others from making, using, offering for sale, or selling his or her invention for a period of 20 years from the filing of the patent application. An invention is any new or useful process, machine, article of manufacture, or composition of matter. An improvement on any of these items also can be an invention. Patent rights are territorial in nature and exist only in the national jurisdictions in which the patentee has applied for and received recognition of his property rights3.
Criteria for Patentability
For any patent to be granted in any geography it should follow the following criteria:
• Invention should be a Patentable subject matter in the US geography
• It should have unity of invention.
• Have Inventive step or Non-Obvious to a person skilled in the art.
• Be capable of industrial application i.e. Utility.
Although most countries in the world apply an absolute novelty requirement (that is, disclosure in any form anywhere in the world before the filing date will prevent the granting of a patent) some countries maintain a double standard of novelty depending on whether the disclosure of the invention has taken place within or outside their territory. In practice, the concept of novelty is narrowly construed by some patent offices, requiring an almost ‘photographic’ disclosure of the invention in a single prior document in order to consider that novelty does not exist [4, 11, 14].
Whether a claimed invention meets the tests of novelty and non-obviousness is determined by comparing it to previously disclosed information in the same field. Defining ‘non-obviousness is one of the most critical aspects of a patent regime, as it determines the level of technical contribution required to obtain a patent and the corresponding limitation on competition. Patent examiners need to consider not only what is disclosed in the prior art but also what a person skilled in the art could consider obvious in the light of such prior art. A person skilled in the art is not just an expert in his technical field but a person who should have some degree of imagination and intuition [4, 15, 16].
Patent protection for pharmaceutical products in the developing world can help to encourage the development of new medicines for diseases that affect these countries, by providing protection for the investments that need to be made by the pharmaceutical companies. Pharmaceutical companies often maintain that patent protection for drugs ensures that they are able to invest billions of dollars into the development of new products, by making sure that they will be able to take advantage of the sales. However, when the ideas that are being protected are medicinal drugs, this can be very controversial. Much of the controversy over pharmaceutical patents relate to the provision of drugs in the developing world [2, 17].
Pharmaceutical products take a very long time to develop and enter market for human use. It takes 10–15 years on average to develop a new dosage form which is clinically proved safety and efficacy from the earliest stages of compound discovery through approval. As a result, significant portions of the patent term for a new drug are lost before a product enters the market. In fact, the average effective patent life for medicines is 11.5 years5, 9. The same is illustrated in the below figure:
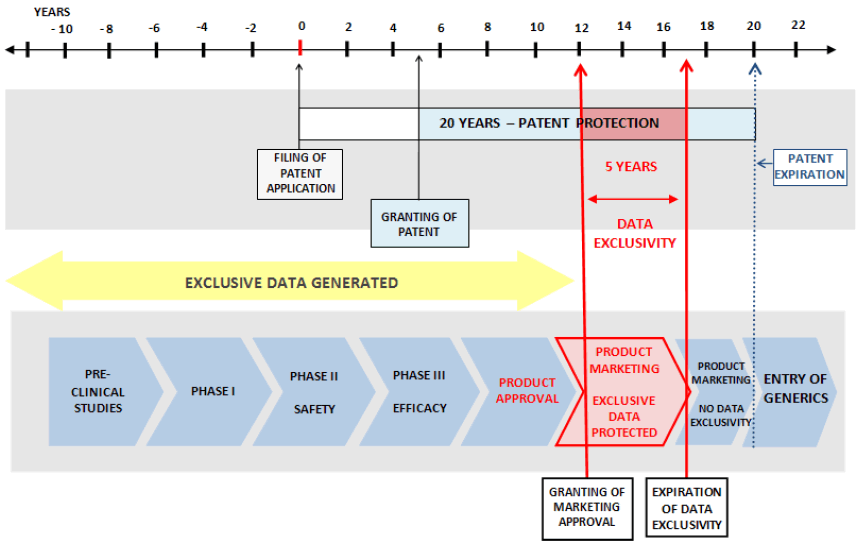
Since initial investment in pharmaceutical R&D is costly, strong patent protection is an important step to provide the opportunity to recoup investments in new products. The extensive cost required to produce a new pharmaceutical product has meant that private sector investment in pharmaceutical innovation has been disproportionately directed to products meeting the needs of patients in developed countries, particularly in the United States, which combines strong patent protection with a market free of price controls. The average cost to develop a new medicine has been estimated at upwards of $800 million [5, 11].
Patents are the legal protection for inventions, including new medicines discovered by research-based pharmaceutical companies. This protection allows a company time to recoup their significant investment in research and development. For a patent to have any commercial value there must be a market for the invention embodied in the patent, which will support the cost of development of the invention and return a profit [5, 15].
In return for such protection, a patent-holder discloses to the world patented research and science underlying the invention. Thus, important scientific information behind a new cancer drug becomes available immediately to researchers worldwide.
The market exclusivity and higher prices are made possible by the patent rights function as a reward for the risk undertaken by those who financed the research and development leading to the new technologies [9, 13].
Patents relating to pharmaceutical inventions
A patent claim relating to a pharmaceutical product may relate to an active ingredient as such independently of or jointly with formulations, salts, prodrugs, isomers, etc., or cover any of these subject matters separately. It may also solely cover a manufacturing process or include both a process and a product. In some countries, as noted below, use-related claims are admissible. The following sections include some considerations for the evaluation of different types of claims that are typical in this area.
In undertaking such evaluation it will be important to bear in mind that while the development of new molecules of pharmaceutical use may encompass various levels of inventive steps, pharmaceutical techniques for the preparation of medicines in different forms and dosages are generally well known and part of the pool of knowledge in possession of a ‘person skilled in the art’. Hence, there is a narrow range of developments that could be considered genuinely inventive in this field in view of the state of the art [4, 13].
Patents relating to formulations and compositions
The same active ingredient may be presented in different dosage forms, for instance, as tablets, capsules, ointment or aqueous solutions for parenteral administration, which in turn can be formulated using different pharmaceutically acceptable excipients [2, 9]. A large number of patents claim formulations of new or existing drugs, often including specifications of dose or concentration, either as the principal claim or in subordination to claims over the active ingredients or their uses. ‘Composition claims’ cover active ingredients and pharmaceutically acceptable carriers or excipients, such as fillers, binders, disintegrants and lubricants. Finally, it should be noted that processes to prepare formulations or compositions are generally well known and routinely applied. Hence, claims over such processes would rarely be inventive [2, 12].
Over the past decade, drug companies have become increasingly dependent on patented blockbuster drugs - patented specialty drugs that generate more than $1 billion in sales annually.
A short comparison of products that lose patent protection:
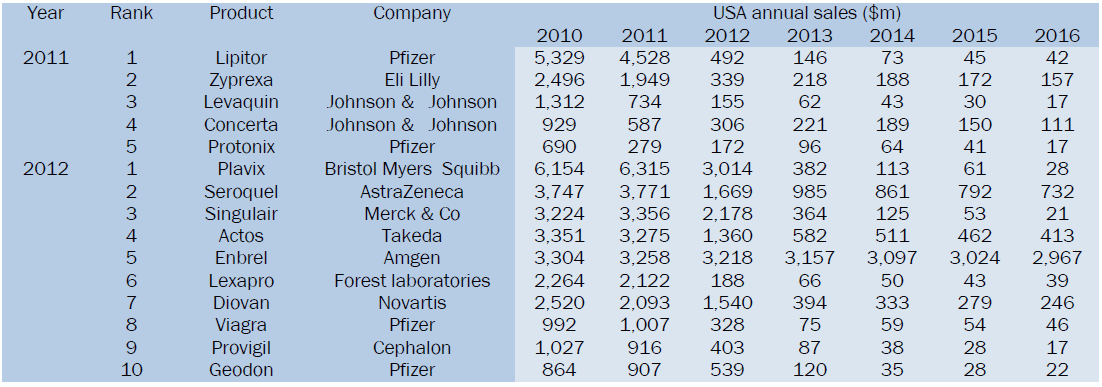
Thirteen US blockbusters will lose patent protection over the next two years, the below data show the challenges facing the pharmaceutical industry. Drugs worth $15.3bn faced generic competition in year 2011-12. $133bn of branded drugs face patent expiry in next six years in the US alone. To highlight Pfizer to bear brunt of patent cliff with $11bn Lipitor expiry in year 2011 [6].
Top products going off-patent in 2011 and 2012
However, when a major blockbuster loses patent protection, generic competition leaves a gaping hole in the top line, abruptly changing a company's greatest strength into its greatest liability.
Let's take a closer look at three blockbuster drugs that will lose patent protection in 2014 [7].
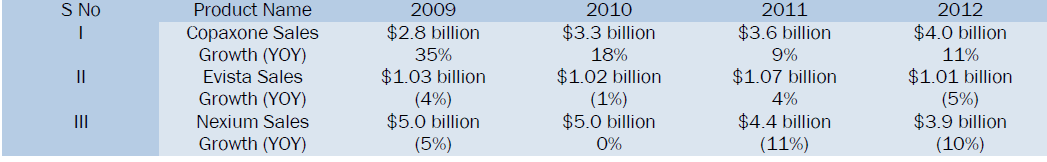
By 2016, medicines that generate sales of $133bn for their manufacturers in the US alone will be exposed to generics [8].
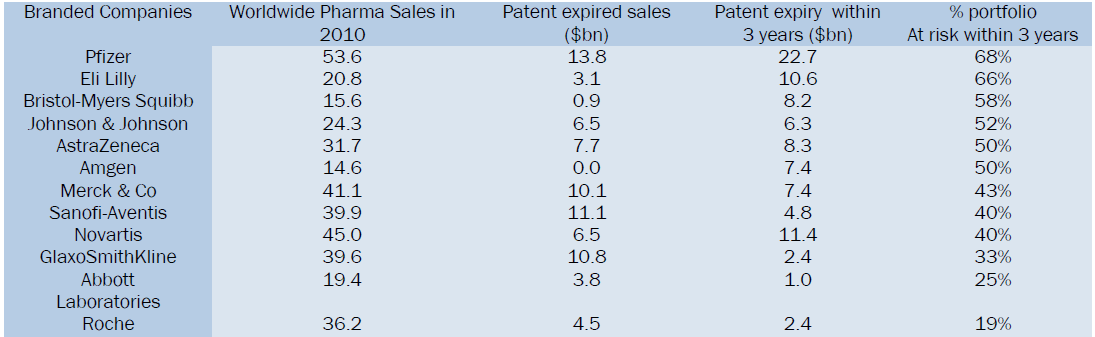
The below table gives a comparison of Branded companies patent expiries consecutively leading in reduction of product sales and further the risk of their product likely to get genericized8.
It is known fact that Patents are Territorial rights and Patent regimes are generally part of national technological and industrial strategies, but is also crucial to design them consistently with public health strategies. Patents over minor developments may be effectively used to discourage or block competition, as generic producers, purchasing agencies and consumers, especially in developing countries, generally lack the substantial technical and financial resources needed to challenge wrongly granted patents or defend against infringement claims. It is very important for branded pharma companies to obtain patent protection for their products in order to garner the investments. Therefore it is widely acknowledged that patents are a fundamental incentive to innovative activities in pharmaceuticals and biotechnology industry, hence it needs to be safeguarded.