ISSN: 2347-7830
ISSN: 2347-7830
Shahram Sharifi1*, Morteza Hosseini1 and Hassan Amini-Rad2
1Department of Chemical Engineering Biotechnology, Noshirvani University of Technology, Babol, Iran
2Department of Civil Engineering, Noshirvani University of Technology, Babol, Iran
Received: 03-Jul-2022, Manuscript No. JEAES-22-68369; Editor assigned: 6-Jul-2022, Pre QC No. JEAES-22-68369 (PQ); Reviewed: 21-Jul-2022, QC No. JEAES-22-68369; Revised: 05-Sep-2022, Manuscript No. JEAES-22-68369 (R); Published: 13-Sep-2022, DOI: 10.4172/2347-7830.10.07.010
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
In this study, for the first time, novel natural coagulant Vicia villosa Roth was used to turbidity removal from water. Vicia villosa Roth is a weed in a wheat field. This coagulant is affordable, eco friendly and local availability. In addition to distilled water, NaCl and NaOH were used as solvents to investigate further and increase coagulation efficiency. A simple extraction method was also used to activate the coagulation agent in the coagulant extract. Synthetic turbid water was obtained by adding kaolin to distilled water and its suspension as stock. Initial synthetic turbidity from 25 NTU to 500 NTU covering the range of low, medium and high turbidity was used for the experiments. The effect of pH 2-12, dose 25-200 mg/l and concentration of solvents with different values were investigated to obtain maximum turbidity removal. Comparison of the performance of different solvents (NaCl>DW>NaOH) showed that 0.5 M NaCl solvent had a higher efficiency for turbidity removal than other solvents. Sedimentation rate was investigated in both turbid water samples based on distilled water and tap water. It was observed that the highest amount of sedimentation occurs in the first 5 minutes (about 94%). The FTIR results in Vicia villosa Roth seeds powder showed the presence of influential factors in the coagulation process (protein, hydroxyl, carboxyl and amino acids). It has the best performance in the pH range of 5 to 7. The highest amount of removal turbidity (99.1%) was obtained for Vicia villosa Roth coagulant at a dose of 125 mg/l and a pH of 5 and the highest initial turbidity, 500 NTU. The results showed the Vicia villosa Roth coagulant has a high ability to turbidity removal from water, which indicates its competitiveness with chemical coagulants.
Natural coagulants; Vicia villosa roth; Turbidity removal; Water
Water is an essential matter for and natural activities. It is therefore vital to outreach human life and ecosystems as part of the hydrological cycle [1]. Water scarcity is growing at an alarming pace and it has become a worldwide concern for sustainable development [2]. Among the treatment technologies, the process that is still widely used in many water and wastewater treatment plants and is one of the oldest treatment processes is coagulation. Coagulation is a process in which smaller particles, which are suspended and colloidal, are transformed into large flocs and removed from the water by destabilizing, binding, and accumulating these particles [3]. Coagulants are available in many varieties. The most commonly used chemical coagulants today are based on alum and iron salts [4]. Despite the proven therapeutic effect of inorganic and synthetic coagulants, the resulting defects have led to the search for natural coagulants [5]. The advantages of natural coagulants that lead to more research in this field include lower cost, environmental friendliness, less sludge volume, less impact on temperature changes and also no need for pH adjustments [6-8]. Natural coagulants are generally obtained from animals, plants, and microorganisms and classified into polysaccharides, proteins, amino polysaccharides, and polyphenols. Classification of plant-based coagulants includes Fruit waste (seeds of Carica papaya, seeds and pollen sheath of Phoenix dactylifera, peels of Citrus sinensis, etc.), Plants Containing Starch (Corn starch, rice starch, etc.), Legumes (Phaseolus vulgaris, Moringa oleifera, Vicia faba, etc.) [9,10]. With all the characteristics and advantages that natural coagulants have over chemical coagulants, but for their application and industrialization, methods and modifications are needed to improve their performance in practice. These methods include integrated/hybrid process; modify the natural coagulant to increase performance; use other types of coagulants with natural coagulants for hybridization; Improve the purification and extraction of these coagulants; and the synthesis of multifunctional coagulants [11]. VVR is a type of annual herb (legume species) that grows well in semi arid regions of the world [12]. Hairy vetch (VVR) was first well known in Argentina in 1950; from that time forward, various naturalized hairy vetch varieties [13]. In the present work, VVR seeds were used as a novel natural coagulant. In addition to high turbidity removal efficiency, which is competitive with chemical coagulants, VVR, In contrast to these coagulants, has features such as safe for health. Environmental friendliness, less sludge volume, local availability, low cost, no need to adjust the pH, maintain efficiency against temperature changes and also non corrosive. In addition to the preparation of coagulation extract, other parameters such as dose, pH and initial turbidity were studied to evaluate the coagulation efficiency. Since the coagulation efficiency strongly depends on the coagulant, the coagulant concentration was able to effectively remove turbidity from the water by the influence of other parameters. To further investigate the coagulant dimensions, coagulation, flocculation and sedimentation processes were examined.
Coagulant preparation
Vicia villosa Roth was harvested from our family wheat field (Plain of Rig region of Lordegan city functions). It is a weed in wheat fields that usually grows in May. The seeds were separated from the cocoon and exposed to the sun for two weeks, and the seeds were dried entirely. The seeds were transported to the laboratory. First, the seeds are thoroughly washed to remove any contaminants, and then we put them in the oven at 100°C for 2 hours to dry completely. Seeds milling was done in two steps; first using a manual mill and then, the Bosch electric mill was used to obtain a finer mill. The powder obtained from the seeds was sieved using a 0.4 mm sifter [14].
Extraction method
Amount of 5000 mg of sifted seeds powder was mixed with 100 ml of solvent for 10 minutes at 500 rpm using a mechanical stirrer to extract the active coagulation agent from the seeds. The solution was then filtered using thick filter paper. Solvents used included Distilled Water (DW), NaCl and NaOH at various concentrations.
Preparation of synthetic turbid water
First, some kaolin (slightly more than 10 mg) was placed at 103°C for 4 hours to remove possible moisture. Then to prepare a stock of kaolin solution, 10 mg of it was added to 1000 ml of distilled water [15]. This stock of kaolin solution was used to prepare water with initial turbidity of 25,50,100 and 500 NTU. Except for the settling velocity part, in which the water sample was distilled water, in all tests, tap water was used as a water sample to synthesize the initial turbidity. These synthetic water turbidities were used for coagulation tests. The reason for choosing these turbidities is that they cover the range of different turbidities of water from low, medium and high. The initial pH was adjusted for the desired amount of synthetic water with 1 mol/l NaOH or 1 mol/l HCl.
Coagulation test
The coagulation activity of the VVR extract was determined using the Jar test. 300 ml of 600 ml beakers were filled with various synthetic turbidities. The beakers were put in splits of the jar test, which was each section equipped with a lamp for lighting. The next step was to add different doses of VVR extracts to the synthetic wastewater sample. This was done in rapid mixing (100 rpm for 4 minutes) and then the speed was reduced to 25 rpm for 25 minutes. After 30 minutes of settling, clear samples were gathered from the top of the beakers and their final turbidities were measured as Tf. Initial turbidity with a similar test was conducted without coagulant and Ti was considered the measured number of turbidity (Tf and Ti was measured using turbidity meter). Turbidity removal was calculated as:
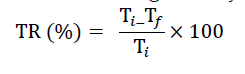
The sedimentation rate of the flocs
The structural properties (size, density, and shape) of the flocs resulting from particle adhesion determine the sedimentation rate. The forces that affect this process are mainly Brownian motion, shear stress, flow turbulence, gravitational force and electrochemical forces [16]. Sticky sediments are different in size, shape and density from non sticky sedimentary particles due to the nature of the particles adhering to each other and the formation of flocs, as a result, their fall rate is also different [17]. A liquid densitometer (hydrometer) was used to measure the sedimentation rate of the flocs. First, the density of suspended particles in water high turbidity (Add stock solution to 1 liter of distilled water) was measured in a 1000 ml graduated cylinder without adding a coagulant. The coagulation and flocculation process was then performed by adding a coagulant into the graduated cylinder using a magnetic stirrer. Immediately at the beginning of the sedimentation process, the hydrometer was placed inside the cylinder and the number shown by the device was read in 15 and 30 seconds of 1, 2.5, 5, 7.5, 15, 30 minutes and 1,2 hours. Finally, it was calculated using the Lau and Krishnappam formula, which states the velocity at which sticky particles fall is related to time:
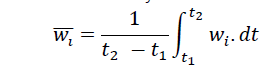
Which obtained by integrating the following relation:
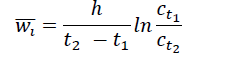
Where wi is the average velocity of the flocs,
c is the concentrations of the suspended solids at times
t1, t2 and h is the flow depth.
Chemical and physical specifications of water samples given in Table 1
| S. No. | Parameters | Unit | Amounts | WHO standards |
|---|---|---|---|---|
| 1 | Turbidity | NTU | 0.1 | <5 |
| 2 | Temperature | ℃ | 24.3 | 12-25 |
| 3 | PH | 7.3 | 6.5-8.5 | |
| 4 | Conductivity | µs/cm | 388 | 1000 |
| 5 | Dissolved oxygen | mg/l | 6.1 | 8.72 |
| 6 | Iron | mg/l | 0.26 | 0.3 |
| 7 | Nitrite | mg/l | 0.02 | <3 |
| 8 | Fluoride | mg/l | 0.32 | >1.5 |
| 9 | Manganese | mg/l | 0.13 | 0.1-0.5 |
| 10 | Chloride | mg/l | 0.35 | <250 |
| 11 | TDS | mg/l | 217 | <1000 |
Table 1. Specifications of water samples.
Effects of various solvents
Various solvents and solutions can extract the active coagulant agents in the seeds, including water and organic solvents and saline and buffering solutions [18]. In this study, in addition to distilled water, different molar concentrations of NaCl (0.01 M to 1.0 M) and NaOH (0.005 M to 0.1 M) were used to improve the extraction process. Of the three solvents used in VVR seeds experiments, NaCl (0.5 M) salt extracts were more effective in removing turbidity. When the NCL concentration exceeds 5 M, the percentage of turbidity removal decreases [19]. At concentrations above 0.5 M, the percentage of turbidity removal began to reduce with increasing NaCl concentration. As the initial turbidity increased, so did the removal percentage. Higher initial turbidity of 500 NTU 0.5 M NaCl showed the highest percentage of turbidity reduction (99.1%). For the minimum initial turbidity of 25 NTU and the medium turbidity of 50 and 100 NTU at 0.5 M NaCl, the turbidity removal percentages were 87.4%, 96.8% and 97.3%, respectively (Figure 1).
Figure 2 shows the effect of NaOH solvent on the activation of coagulants. It shows the percentage of turbidity removal using different concentrations of NaOH to extract coagulant agents from seed in different amounts of primary turbidity of synthetic wastewater. The NaOH concentration range used was 0.005 to 0.5 M. As the NaOH concentration increased, the coagulation efficiency of the effluent also increased until it reached its maximum at 0.05 M. A similar behavior was observed in turbidity removal for all initial turbidity ranges of the studied wastewater. Soluble at concentrations of NaOH above 0.05 M became found a viscous state due to a decrease in the solubility of the coagulant protein at high concentrations of NaOH. Therefore, coagulation activity decreased when it exceeded this value. After reaching the optimum level, the reduction of coagulation activity showed that some protein might be denatured at a concentration of NaOH higher than 0.1 M, which reduces the solubility of the protein in the crude extract solution [20]. 0.05 M NaOH, like 0.5 M NaCl with high turbidity, i.e., 500 NTU showed the highest amount of turbidity removal (81.3%). In the initial turbidity of 25, 50 and 100, 41.7%, 62.6% and 70.4% of the turbidity removal values were obtained, respectively.
Figure 3 shows that of the three solvents used to activate the VVR coagulant, NaCl had the highest performance. Using NaCl, distilled water and NaOH solvents, 99.1%, 98.2% and 81.3% were obtained for more effective coagulation of turbidity removal from synthetic wastewater, respectively. Different initial turbidities (25, 50, 100 and 500 NTU) were prepared as water samples according to section 2.3. At the highest initial turbidity, 500 NTU, the highest turbidity removal occurred.
Effects of pH and coagulant dosage
Changes in water pH affect the surface charge of coagulants as well as the stabilization degree of the suspension [21]. The effect of water pH on turbidity removal was investigated by VVR over a wide pH range of 2 to 12. Figure 4 shows the effect of pH on turbidity removal using different solvents of NaCl, NaOH and distilled water. With increasing pH, the turbidity removal percentage increased until it reached its maximum value at pH 5. The highest turbidity removal was obtained in NaCl, NaOH and distilled water solvents at pH 5 of 99.1, 81.2 and 98.4, respectively. After pH 5, the turbidity removal percentage decreased, which was imperceptible until the neutral pH. The average percentage of turbidity removal in the pH range of 5 to 8 indicates that this coagulant performs better in this range.
Coagulant dosage is also one of the factors that contribute to coagulation activity. For this purpose, to investigate the effect of coagulant dose on coagulation activity, a concentration range between 25 and 200 mg /l is used for water treatment. Figure 5 showed with increasing coagulation dose, the turbidity removal percentage for 0. 5 M NaCl, 0.05 M NaOH and DW solvents increased. The maximum amount of turbidity removal occurred at a dose of 125 mg /l. Determining the optimum amount of coagulant for optimum treatment performance as well as reducing the cost of dosing and sludge formation is significant [22].
Settling time and fall velocity of flocs
Settling time also has a significant and quantitative effect on coagulation efficiency. Settling time is performed using the optimum dose of coagulant of 125 mg/l to observe the turbidity removal efficiency at different settling times. Initial turbidity of 100 and 500 NTUs was used for this purpose and allowed to settle down from 5 min to 1 h. As shown in Figure 6, 93.82% turbidity was removed during the first 5 minutes, which is a significant number to the time elapsed. At the initial treatment time, the maximum amount of active proteins is present in the aqueous solution, which increases activity [23]. After 15 minutes, the removal value reached 96.57%; as the elapsed time increased, the amount of turbidity removal advanced until in the 30th minute, which is the standard time for the sedimentation process, the turbidity removal percentage reached 97.26%. After that, the amount of removal did not increase significantly due to the decrease in turbidity and coagulant concentration (Figure 6).
Figure 7 shows the sedimentation average velocity of the flocs over time (Under optimal conditions, acidic pH and a dose of 125 mg/l). The maximum sedimentation average velocity is related to the initial 5 minutes, equal to 6.43 cm/min, after which the rate begins to decrease. The reason for the high rate of settling in the early times is due to the increase congestion of the flocs; larger flocs drop faster due to the force of gravity and also flocs collide with each other during the fall and also pull the smaller flocs down. According to the reasons given, as shown in Figure 7, the average sedimentation rate is higher in the first 5 minutes, which is a confirmation of the removal percentage over time (Figure 6), which showed that the highest removal rate of about 94% occurred in the first 5 minutes of settling time. After that, the average sedimentation rate gradually approached zero, due to a significant decrease in the concentration of flocs (Table 2).
| Extraction method | Blending | |||||
|---|---|---|---|---|---|---|
| Mechanical stirrer(blending time) | 10 min | |||||
| Extracting solvent | 0.5 M NaCl | DW | 00.5 M NaOH | |||
| Turbidity removal (%) | 93.82 | 96.9 | 99.1 | 98.2 | 81.3 | |
| Sludge volume (ml sludge/ml treated water) | 0.0035 | 0.0041 | 0.0048 | 0.0045 | 0.0026 | |
| Sedimentation time(min) | 5 | 15 | 30 | 60 | ||
| Sedimentation average velocity(cm/min) | 6.43 | 5.18 | 4.33 | 3.25 | ||
Table 2. Summary of experiments performed.
FTIR analysis
Proteins, lipids and carbohydrates are macromolecules that combine to form natural coagulants. Mostly, the primary building blocks are the polymer of amino acids and polysaccharides [24]. Hairy vetch (Vicia villosa Roth) has high protein content [25]. So it is rich in amino acids. The presence of hydroxyl and amino (âÃâ¬ÃÂNH2, âÃâ¬ÃÂCOOH, and organic R group) functional groups in natural coagulants contribute to coagulation capability. The intensity of the spectrum at about 1400 cm−1 indicates carboxylic COOH bending [26]. There is a proline at the 1454 cm-1 band [27]. Mainly from N-H deformation and C-N stretching in -CO-NH- bond, 1538 cm-1 peak is obtained, which shows the characteristics of amide II [28]. The vibration at 1648 cm−1 was assigned to the C-O stretch of amide I in proteins (mainly α-helix components of proteins) [29]. Stretching vibrations of CO2 at about 2364 cm−1. The absorption bands at 2927 cm−1 due to aliphatic CH stretch. The strong peak observed at wavelengths between 4000 and 3000 cm-1 was attributed as the surface of the hydroxyl group (-OH stretching group) (Figure 8).
The use of Vicia villosa Roth seeds as a coagulant showed a significant effect in removal turbidity to obtain drinking water. It also has advantages over chemical coagulants. The use of the simple extraction method to extract the active coagulation agent from the seeds led to the formation of acceptable flocs and resulted in a reasonable settling rate and high turbidity removal. The maximum reduction of turbidity was obtained in water with very high turbidity and at the same time, it was noticeable in the range of medium and high turbidity. It was also acceptable in removing water turbidity with low turbidity. According to the desired results, as a sustainable and natural source, VVR seeds can be used to purification turbid water.
[Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]
[Crossref] [Googlescholar] [Indexed]