ISSN: 2347-7830
ISSN: 2347-7830
Department of Analytical Chemistry, International Institute of Bio-technology and Toxicology (IIBAT), Padappai, Chennai 601 301, Tamil Nadu, India
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
Phototransformation of the herbicides fenoxaprop-p-ethyl and tepraloxydim in natural sunlight was investigated in Milli-Q water and in three different aqueous buffer solutions of pH 5.0, 7.0 and 9.0 at 1.0 μg/mL and 2.0 μg/mL levels. Aliquots of water samples were collected in predetermined intervals and analyzed by a validated HPLC-UV. The method has the limit of quantification 0.01 μg/mL. The influence of cations (Fe2+, Cu2+, Co2+, Ni2+, Mn2+ and Zn2+) and anions (SO42-, Cl-, ClO4-, CO3- and HCO3-) in the photolytic degradation process were studied by adding different concentrations of ions (10-1M, 10-2M and 10-3M) in Milli-Q water. The effect of aeration was also studied in water. A study in dark was conducted at 25°C to understand the hydrolytic behavior of the pesticides under sterile condition. Complete mineralization of residues was confirmed by LC-ESI-MS/MS. A study was conducted to know the impact of residues on the growth of fresh water green alga (Pseudokirchneriella subcapitata).
Alga, Fenoxaprop-p-ethyl, Herbicide, HPLC-UV, LC-ESI-MS/MS, Phototransformation, Tepraloxydim.
The extensive use of crop protection chemicals in agriculture has dramatically increased the various pesticide residues in food, agricultural and in environmental samples [3]. The contamination of surface water and sediments of non-point sources with the pesticide residues either in the free form or as bound residues is a major environmental concern [1]. Pesticides enter into natural water bodies by direct application and by leaching from soil and vegetation. Many of these chemicals present in aqueous media can undergo photochemical transformation with direct sunlight or indirect photoreaction. To assess the role of these processes on the behavior and fate of pesticides in natural water systems, photochemical studies in aqueous solution over a wide range of environmental conditions are needed [8]. The photochemical degradation of residues by sunlight is the primary route for the removal of pesticides at a greater extent in soil, plant, water and in other environmental strata.
The photochemical transformation is one of the main routes for the dissipation of pesticides and other xenobiotics under natural conditions [11]. Herbicides are widely used in crop protection to control a wide variety of weeds. Herbicide run-off and leaching down the soil profile have become a serious environmental problem and a primary source for polluting surface and ground water. Fenoxaprop-p-ethyl [(R)-2-[4-(6-chloro-1,3-benzoxazol-2-yloxy)phenoxy]propionic acid] is an aryloxyphenoxypropionate herbicide, which is a post-emergence herbicide used for the control of grass weeds in soybean, potato and vegetables. The trade names are Furore Super and Whip Super. Tepraloxydim [(EZ)-(RS)-2-{1-[(2E)-3-chloroallyloxyimino]propyl}-3-hydroxy-5-perhydropyran-4-ylcyclohex-2-en-1-one] is a cyclohexanedione oxime herbicide which is a post-emergence herbicide used for the control of grasses and broad leaved weeds in soybean and maize. The trade names are Aramo and Neto. Photolysis, hydrolysis, aquatic metabolism, leaching and field dissipation studies are used to determine the potential of a pesticide persistence and their leaching. Photolysis and hydrolysis are two main degradation pathways of pesticides in the environment [18]. High Performance Liquid Chromatography Tandem Mass Spectrometry (HPLC–MS/MS) method was developed to evaluate the residual level and dissipation rate of fenoxaprop-P-ethyl and its metabolite (fenoxaprop-P) in the soil and wheat [19]. Hydrolysis of fenoxaprop-p-ethyl was studied in aqueous buffer solutions at pH ranging from 4.0 to 10.0. The degradation kinetics, followed first-order kinetics [12]. The effects of the photodegradation on its toxicity evolution were investigated and the photodegradation pathway of fenoxaprop-p-ethyl was also elucidated. More toxic and photoresistant products were generated from photolysis of the herbicide [13]. An analytical method for the determination of fenoxaprop-p-ethyl and its main metabolite in the edible fractions of rice was developed and validated. Parent compound was extracted in acetonitrile and determined by gas chromatography with a mass spectrometer detector and its metabolite by liquid chromatography tandem mass spectrometry. The environmental persistence and fate of fenoxaprop-p-ethyl) was investigated under laboratory and field conditions. In the field, it dissipated rapidly from both water and soil, with half-lives in water was less than 4 hour and in soil residues went to below detectable level within 6 days [14]. The photolysis half-life in sterile, distilled water was 269-19 hour; in field water a combination of microbial and photochemical reactions resulted in a half-life of 29-2 hour [2]. A method for direct analysis of tepraloxydim and its main metabolites in water was established by solid-phase extraction and HPLC-UV determination. It was observed that chlorine added to tap water as disinfectant eliminates the possible residues of tepraloxydim [17].
A significant fraction of applied pesticides enters the soil, sediments and water where it can undergo various transformation processes which can lead to formation of stable transformation products in the aquatic environment [5]. The different transformation processes may significantly change the toxicity in comparison to the parent compound [9]. Pesticides play an important role in agricultural practices. Escalating use of pesticides can affect the non-target organisms such as alga. Therefore, their potential effects on the aquatic primary producers are particularly important and have to be studied in ecotoxicological experiments [4]. Alga is an important component of the primary production and detrimental effects in these organisms may affect the entire food chain. Pseudokirchneriella subcapitata (formerly Selenastrum capricornutum) is a unicellular chlorophyceae alga that was widely used in studies of pollutants effects and recommended by regulatory national and international agencies as a test organism [7]. Alga populations are employed for estimating the toxicity of wastes and receiving water. Alga growth inhibition tests are relatively sensitive bioassay for a large variety of chemicals. Alga is expected to be more sensitive to herbicides than other aquatic organisms because the targets of these compounds are usually either photosynthesis or energy transport enzymes [10]. The alga growth study is an indication of understanding the complete photolysis of pesticides and the impact of break down products if any.
However the data pertaining to the fate of fenoxaprop-p-ethyl and tepraloxydim in water and the information on the effect of residues on the growth of fresh water green alga is scarce. In view of paucity of information, present study was conducted with an objective to understand the photolysis of herbicides belonging to different class/groups with varied KOC values and GUS values having the potential to drift and contaminate the aquifers during run off, to understand the formation of photolysis products, their significant stability and impact on aquatic species.
Reference analytical standards of purity fenoxaprop-p-ethyl 99.3%, tepraloxydim 99.8%, fenoxaprop-p-ethyl 8% EC and tepraloxydim 5% EC used were obtained from the pesticide market. Stock solution (1000 μg/mL) of fenoxaprop-p-ethyl and tepraloxydim was prepared in acetonitrile. Different known concentrations of calibration solutions (2.0, 1.0, 0.5, 0.1, 0.05, 0.01 and 0.001 μg/mL) were prepared in acetonitrile by diluting the stock solution. The standard solutions were injected in High Performance Liquid Chromatography (HPLC) and peak area was measured. A calibration curve was plotted for concentration of the standards injected versus area observed and correlation coefficient was determined. Acetonitrile HPLC gradient grade and ortho phosphoric acid were obtained from Merck India Limited. Merck India Limited supplied the following chemicals ferrous sulfate, copper sulfate, cobalt nitrate, nickel sulfate, manganese sulfate, zinc sulfate, sodium sulfate, sodium chloride, sodium perchlorate, sodium carbonate and sodium bicarbonate. All the chemicals used were analytical reagent grade. Distilled water was purified using by passing through the Milli-Q apparatus (Millipore, Bedford, MA, USA).
The quantification of residues of fenoxaprop-p-ethyl and tepraloxydim was done by High Performance Liquid Chromatograph using a Shimadzu prominence equipped with two pumps (model LC-20AT), oven (CTO-20A), Ultra Violet detector (SPD-20A), and a C18 reverse phase column (25 cm length x 4.6 mm i.d x 5 μ particle size, Phenomenex). Eluent was a mixture of water and acetonitrile (70:30 v/v) for fenoxaprop-p-ethyl and water acidified with orthophosphoric acid (pH 3.0), acetonitrile (70:30) for tetepraloxydim with 1.0 mL/min flow rate; detection was at 230 nm for fenoxaprop-p-ethyl and 254 nm for tepraloxydim with an injection volume of 25 μL.
High Capacity Ion Trap (HCT plus) LC-MS/MS system supplied by Bruker Daltonik, GmbH, Germany was used to identify the formation of breakdown products during the study. From the pressurized air using a Nitrox UHPLCMS nitrogen generator, drying gas and the nebulizing gas nitrogen was generated. The nebuliser gas nitrogen flow was fixed at 10 L/min. For MS/MS mode operation, helium was used as collision gas. A capillary voltage of 4.5 kV was used in positive ionization mode. The interface temperature was aet as 340 °C. The scan range was 50 - 400 m/Z. Agilent 1200 HPLC system with Zorbax SB C18 column (5 μm particle size, 4.6 mm i.d., 75 mm length) with gradient elution of 0.25 ml per minute. 0.1% formic acid in acetonitrile as mobile phase A and 0.1% formic acid in Milli-Q water as mobile phase B were used as a mobile phase for the separation of fenoxaprop-p-ethyl and tepraloxydim. The injection volume used for the analysis was 10 μL.
Recovery study was conducted by fortifying three different concentrations of fenoxaprop-p-ethyl and tepraloxydim (0.01, 0.05 and 0.1 μg/mL) in Milli-Q water, acidic, neutral and basic buffer solutions. The Limit of Quantification (LOQ) was established based on the recovery study and signal to noise ratio with minimum of 10:1. From the linearity of the method and based on signal to noise ratio 3:1, Limit of Detection was also determined.
The photolysis of fenoxaprop-p-ethyl and tepraloxydim in water was studied by spiking two different concentrations of fenoxaprop-p-ethyl 8% EC and tepraloxydim 5% EC at 1.0 μg a.i./mL (T1) and 2.0 μg a.i./mL (T2) along with control (T0). The study was conducted using Milli-Q water and in three different buffer solutions of pH 5.0, 7.0 and 9.0. Three replicates were prepared at each fortification level along with the control sample for comparison. All the samples were exposed to sunlight till the dissipation gets completed. The dissipation of residues was monitored by collecting and analyzing the aliquots of water samples at pre-determined intervals using different validated analytical techniques. The day temperature of the water samples during the period varied between 26 to 45 °C. The intensity of the sunlight was measured during the exposure using LUX meter.
To identify the hydrolysis behavior of fenoxaprop-p-ethyl and tepraloxydim, a set of spiked samples were kept in dark at 25°C under sterile conditions. The samples were collected at pre-determined intervals for analysis. To analyze the microbial impact on degradation during hydrolysis sterile water was used.
All the samples collected at different time points were directly injected into HPLC after filtration with 0.2μm PTFE filter.
To determine the influence of cations and anions in the photolytic degradation process, the water samples fortified with different concentrations of metal salts (10-1M, 10-2M and 10-3M). The metal ions used were Ferrous sulfate (Fe2+), Copper sulfate (Cu2+), Cobalt nitrate (Co2+), Nickel sulfate (Ni2+), Manganese sulfate (Mn2+), Zinc sulfate (Zn2+) and anions used were Sodium sulfate (SO42-), Sodium chloride (Cl-), Sodium perchlorate (ClO4-), Sodium carbonate (CO3-) and Sodium bicarbonate (HCO3-). After addition of metal ions, the solution was mixed well, fortified with known concentrations of herbicides and kept under direct sunlight. Aliquots of the water samples were withdrawn at regular time intervals, filtered with 0.2μm PTFE filter and analyzed by HPLC-UV.
The aerated system was compared with un-aerated system to know the effect of aeration in the photolytic degradation process. In aerated and un-aerated systems the water samples were spiked with formulation of concentration 1 μg/mL and exposed to sunlight. The degradation of residues was analyzed by injecting aliquot of sample solutions in HPLC-UV at pre-determined intervals.
The effects of fenoxaprop-p-ethyl 8% EC and tepraloxydim 5% EC on green alga, Pseudokirchneriella subcapitata during a 72 hours exposure period was studied according to methods described in the OECD No.: 201 guideline [15]. Test species was Pseudokirchneriella subcapitata was purchased from the culture collection of alga at the University of Gottingen. The test duration was 72 hours and the temperature was maintained in shaker incubator was 22 ± 2°C. The light intensity and pH were kept at 6000-8000 LUX and 8.1±0.1, respectively. All the flasks in the shaker incubator were shaken continuously at about 110-120 rpm. The tested dosage was 1.0 μg/mL. All the conical flasks were randomly repositioned daily in the shaker incubator during the 72 hours study period.
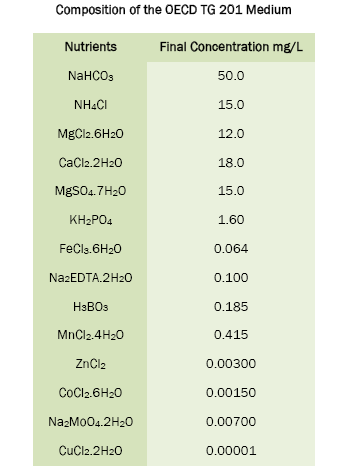
Nutrient stock solutions were prepared by dissolving a known quantity of each nutrient in 25 mL of de-ionised water. The nutrient stock solutions were transferred into amber-colored bottles and stored in the refrigerator for one month.
One day prior to inoculation, required amounts of OECD TG 201 medium was prepared by adding a known volume of each sterile nutrient stock solution into a sterile beaker. The OECD TG 201 medium was then brought to final volume with de-ionised water and the pH was adjusted from 6.88- 8.13 using 0.1 N NaOH. After measurement of pH, the OECD TG 201 medium was sterilized through 0.20 μm pore size sterile membrane filters under aseptic conditions. After filtration, the OECD TG 201 medium was stored in glass bottles, maintained under aseptic conditions. On inoculation day, the pH of OECD TG 201 medium was checked and adjusted from 7.42 - 8.12 using 0.1 N NaOH and used for the experiments.
The stock solution was prepared by dissolving a known amount of each herbicide formulation in two different 100 mL volumetric flasks. To the flasks 25 mL of Milli-Q water was added and sonicated the contents for 5 minutes, allowed the flask to settle at room temperature for 1 hour then made up to the mark using Milli-Q water.
The working solution of each herbicide of 10 μg/mL concentration in 500 mL volumetric flask was prepared by simple dilution and exposed to sunlight.
Three days before initiation of the study pre-culture of Pseudokirchneriella subcapitata was prepared. The inoculated flasks were kept in the shaker incubator with rpm of 114-118 and maintained with continuous illumination of 6842 - 6963 LUX light intensity at 22.3 to 23.7°C for three days. After three days, the culture was examined under a microscope and checked for any abnormality or microbial contamination.
About 35 mL of 10 μg/mL concentration of each herbicide was diluted with 315 mL of OECD medium (1μg/mL). The pH was measured and adjusted to 8.1± 0.1 using 0.1N NaOH. About 100 mL of the test solution was transferred to 250 mL Erlenmeyer flasks. Three replicates were made at each concentration and control. The control and treated flasks were inoculated with 100 μL of Pseudokirchneriella subcapitata from the pre-culture to obtain an initial cell concentration of approximately 1 × 104 cells per mL. The cell counts of Pseudokirchneriella subcapitata (cells/mL) were made by visual counting using an Improved Neubauer’s Haemocytometer under illumination of the microscope at 24, 48 and 72 hours after inoculation. The pH was recorded at test termination.
The yield was calculated using the following formula
Y i – j = Xj - Xi
Where,
Y i – j = biomass from the start of the test to the end of the test
Xi = biomass (cells/mL) at time i (0 hour)
Xj = biomass (cells/mL) at time j (72 hours)
The percent inhibition of the yield (% Iy) at test concentration was calculated as,
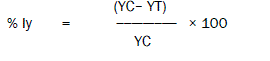
Where,
% Iy = percent inhibition of yield
Yc = mean value for yield in the control group
YT = value for yield for the treatment replicate
The following criteria are to be fulfilled to validate the test. The biomass in the control flasks should be increased exponentially by a factor of at least 16 times (OECD 201 guideline) within the 72 hours test period. The percent coefficient of variation (% CV) for section by section specific growth rate in the controls (0-24 hours, 24-48 hours and 48-72 hours) was less than 35% as per the OECD guideline. As per the OECD guideline the percent co- efficient of variation of average specific growth rate during the whole test period (0-72 hours) in control flasks was less than 7 %.
A good linearity was achieved with a correlation of coefficient of 0.9993 for fenoxaprop-p-ethyl and 0.9999 for tepraloxydim. The limit of detection (LOD) was determined, based on the lowest level of standard concentration detected and was found to be 0.001 μg/ml. The efficiency of the method was evaluated by spiking Milli-Q water and three different aqueous buffer solutions with fenoxaprop-p-ethyl and tepraloxydim standard solutions at various levels (0.01, 0.05 and 0.1 μg/ml). Recovery values for Milli-Q, acidic, neutral and basic buffer solutions ranged from 87% to 91%. All of these values of recovery indicated good method of accuracy and repeatability.
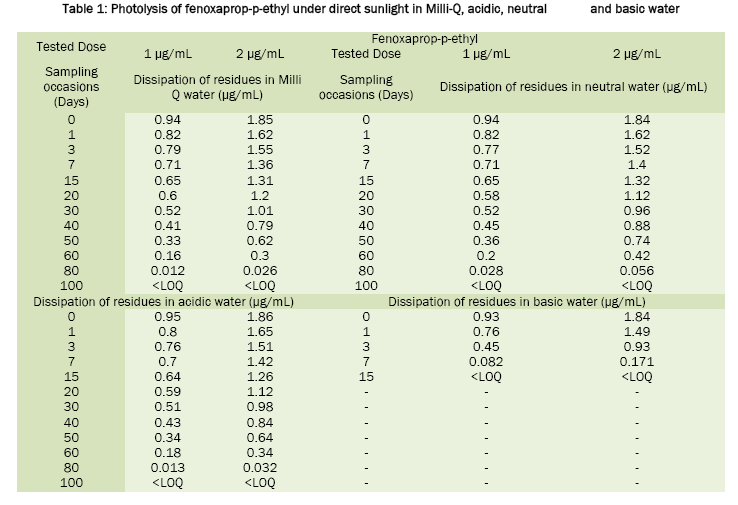
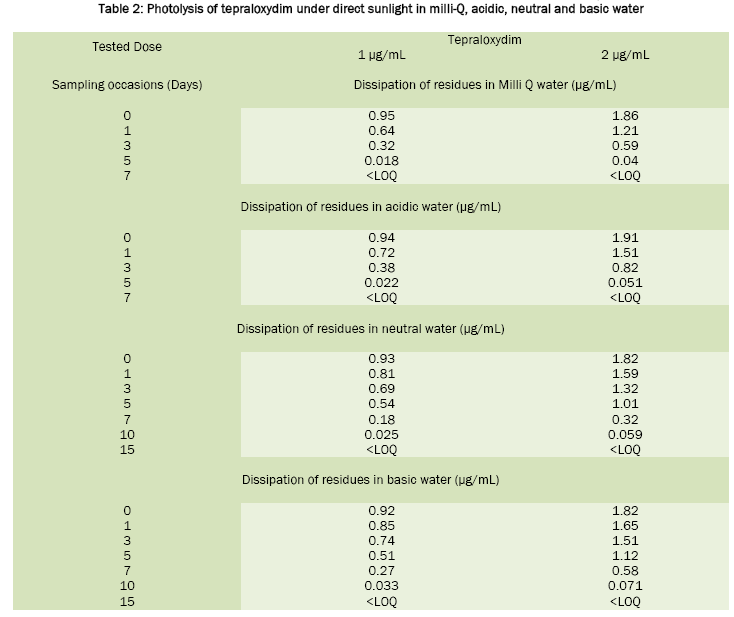
Fenoxaprop-p-ethyl was stable in sunlight; the calculated half life value was around 20 days in neutral water, 17.6 days in acidic water and 16.8 days in Milli-Q water. In basic water it was found to be unstable and has DT50 2.0 days. For tepraloxydim, the half life values were 2.3 days in basic water, 2.1 days in neutral, 1.0 day in acidic and 0.92 day in Milli-Q water. Under the influence of sunlight, the dissipation of fenoxaprop-p-ethyl and tepraloxydim in aqueous buffer solutions was presented in Table 1 and Table 2. Chromatograms of fenoxaprop-p-ethyl and tepraloxydim were presented in Figure 1 and Figure 2.
A study in dark was conducted at 25°C to understand the hydrolytic behavior of the herbicides under sterile condition. The degradation was found to be fast under direct sunlight when compared to dark. The calculated half life values of fenoxaprop-p-ethyl were 38.4 days in Milli-Q water, 40.9 days in acidic water and 43.4 days in neutral water. In basic water it was degraded quickly and the DT50 value was 9.7 days. The herbicide tepraloxydim was stable in basic water (DT50 38.1 days) compared to neutral (DT50 26.3 days), acidic (DT50 11.1 days) and Milli-Q water (DT50 10.1 days). Dissipation curves of fenoxaprop-p-ethyl and tepraloxydim under direct sunlight and in dark was presented in Figure 3.
Anions (SO4 2-, Cl-, ClO4-, CO3- and HCO3-) positively enhanced the degradation of residues. Carbonate radical contributed to the degradation of pesticides with second order reaction [6]. Dissipation rate varied with the use of different cations. The presence of iron, copper and zinc enhancing the degradation rate of fenoxaprop-p-ethyl in the order Cu ≥ Zn ≥ Fe, because they react with different species and produce a path of lower activation energy and allows the reaction to proceed at a faster rate. Nickel has no significant influence on the dissipation rate but manganese and cobalt decrease the dissipation rate and its orders Mn ≥ Co.
The degradation of residues was rapid while aerating the water samples. While aerating the water, the residues of fenoxaprop-p-ethyl and tepraloxydim were gone to below the limit of determination on 100th day and 7th day respectively. In the absence of aeration the degradation was slow and below detectable level was observed by 140th day for fenoxaprop-p-ethyl and 15th day for tepraloxydim. This was to enrichment of the amount of dissolved oxygen and formation of oxonium ions accelerating the degradation of fenoxaprop-p-ethyl and tepraloxydim in water.
The rate constant k [16] was calculated from the dissipation of fenoxaprop-p-ethyl and tepraloxydim with time using the following first order rate equation.
k = ln (a/a-x)/dt
Where dt is the time interval between t1 and t2 and a, x are the concentration of residues at times t1 and t2 respectively. A plot of concentration versus rate with R2 value 1.000 indicates first order kinetics in dissipation. The rate constant was calculated from the first order rate equation.
The herbicide fenoxaprop-p-ethyl eluted at 21.0 minutes and showed a molecular ion peak at m/Z 362 and the major fragment ions at 347, 329 and 288. The breakdown products eluted at 14 to 19.0 minutes. The break down products identified in water samples has the molecular ion peaks at m/Z 334 and 211. The fragment ion peaks obtained at m/Z 288, 244 and 209 for breakdown product having the molecular ion peak of m/Z 334. The fragment ion peaks obtained at m/Z 193, 181 and 123 for breakdown product having the molecular ion peak of m/Z 211. The herbicide tepraloxydim eluted at 19.4 minutes and showed a molecular ion peak at m/Z 343 and the major fragment ions at 250, 222 and 166. The breakdown products eluted at 14 to 18.0 minutes. The break down products identified in water samples has the molecular ion peaks at m/Z 238, 250 and 328. The fragment ion peaks obtained at m/Z 154 and 126 for breakdown product having the molecular ion peak of m/Z 238. The fragment ion peaks obtained at m/Z 194, 166 and 177 for breakdown product having the molecular ion peak of m/Z 250. The fragment ion peaks obtained at m/Z 295, 224 and 183 for breakdown product having the molecular ion peak of m/Z 328. The method has the limit of quantification 0.1 μg/L. Representative LC-MS/MS spectra of fenoxaprop-p-ethyl, tepraloxydim and its breakdown products are represented in Figure 4 and Figure 5.
The initial and final pH of OECD TG 201 medium (Control) were 8.09 and 7.91. The initial pH of the test item concentration (1.0 μg/mL) were in the range of 8.10 - 8.16 and the pH was recorded with the range of 7.31 - 7.40 at test termination. All the flasks were incubated in the shaker incubator during the study period. Temperature, light intensity and rpm of the shaker incubator were recorded daily. The temperature was recorded with the range of 21.5 to 23.6°C, the light intensity was recorded with the range of 6796 to 6882 LUX and the rpm was 112 – 117.
The initial cell count was 1 × 104 (10000) cells/mL in the control flask. During the 72 hours study period the average final cell count in the control at 0th, 7th, 17th 40th, 100th and 120th were 159×104, 109×104, 54×104, 57×104 41×104 and 36×104 cells/mL respectively. The cells of Pseudokirchneriella subcapitata were increased by approximately 159, 109, 54, 57, 41 and 36 times in the control at 0th, 7th, 17th 40th, 100th and 120th days, respectively. The percent coefficient of variation (% CV) for section by section specific growth rate in the controls (0 - 24 hours, 24 - 48 hours and 48 - 72 hours) was 10.59%, 18.15%, 25.64%, 22.71%, 22.05% and 20.18% at 0th, 7th, 17th 40th, 100th and 120th days respectively (less than 35% as per the OECD guideline). The percent co-efficient of variation of average specific growth rate during the 72 hours test period in replicate control flasks was 0.7%, 1.8%, 3.5%, 2.5%, 4.1% and 3.9% at 0th, 7th, 17th 40th, 100th and 120th days respectively (less than 7 % as per the OECD guideline). The results of the present study were validated by the above findings.
Before exposed to sunlight 88% inhibition for fenoxaprop-p-ethyl and 93% inhibition for tepraloxydim was observed at 1.0 μg/mL concentration level. The fenoxaprop-p-ethyl dissipated to below detectable level at 100th day and tepraloxydim for 7th day when analysed in HPLC, whereas 15% inhibition for fenoxaprop-p-ethyl and 78% for tepraloxydim was observed by cell count. The inhibition was due to the presence of persistent metabolites of fenoxaprop-p-ethyl and tepraloxydim in water samples, which was confirmed by the LC-MS/MS analysis. The breakdown products identified on this occasion was fenoxaprop and ethyl 2-(4-hydroxyphenoxy) propanoate. For tepraloxydim the inhibition further dropped to 18% on 17th day. The inhibition was due to the formation of 2-[(1E)-N-{[(2E)-3-chloroprop-2-en-1-yl]oxy}propanimidoyl]-5-(tetrahydro-2H-pyran-4-yl)cyclohex-1-en-1-ol and 2-ethyl-6-(tetrahydro-2H-pyran-4-yl)-6,7-dihydro-1,3-benzoxazol-4(5H)-one. Analysis of 40th day samples showed no sign of inhibition in the growth of green alga and the growth was on par with the control. No sign of inhibition in the growth of green alga was observed on 40th and 120th day samples. The growth of green alga was on same level with the control for fenoxaprop-p-ethyl and tepraloxydim.
Investigations were made on the photolytic degradation of herbicides fenoxaprop-p-ethyl and tepraloxydim in Milli-Q water and in three different aqueous buffer solutions of pH 5.0, 7.0 and 9.0. The degradation pattern of the fenoxaprop-p-ethyl and tepraloxydim was compared with the hydrolysis study. The pH influenced the degradation of herbicides to a greater extent owing to the formation of hydroxyl radicals. The degradation rate of fenoxaprop-p-ethyl in basic water was rapid. The data generated clearly showed that the photolysis of the fenoxaprop-p-ethyl and tepraloxydim in water under direct sunlight was rapid when compared to hydrolysis at 25°C. Dissipation kinetics of the herbicides was investigated under photolytic and hydrolytic conditions and the degradation followed first order. The rate of degradation was influenced by the cations (Fe2+, Cu2+, Co2+, Ni2+, Mn2+ and Zn2+) and anions (SO42-, Cl-, ClO4-, CO3- and HCO3-). This can be attributed to the formation of various radicals, which absorb light and contribute to the photolytic reaction. The metal cations and nitrate (λmax = 303 nm) absorb light and undergo homolysis to produce reactive oxygen species (ROS) such as •OH and reactive nitrogen species (RNS) such as NO• and NO2•. These reactive species facilitate the degradation of herbicides. Among the cations iron, copper and zinc increased the degradation rate, manganese and cobalt decreased the degradation rate, whereas nickel did not have any influence on the degradation. All the anions influenced the degradation rate to the same extent. The presence of some traces of Fe2+ in leaching water can contribute to the degradation following Photo-Fenton reaction. The presence of Cu2+ and Zn2+ have a catalytic effect on the degradation of the pesticides by trapping photo generated electrons and thereby reducing e−/hυ recombination.
The data generated in the degradation studies revealed that the degradation was comparatively fast in aerated water than un-aerated water. This effect was attributed to the enhancement of dissolved oxygen content in water samples which contributed to the formation of oxonium ions facilitating rapid degradation. The breakdown products of herbicides in water, under photolytic conditions were identified and confirmed by LC-MS/MS.
Impact of pesticide residues on the growth of green alga Pseudokirchneriella subcapitata revealed, initially the parent induced higher degree of toxicity. Metabolites were also toxic, but the toxicity gradually declined in due course, as the metabolites were disappearing due to mineralization and there was no toxicity at the end of the experimental period which is attributed to complete mineralization of breakdown products. The results revealed that photolysis can result in the formation of breakdown products which could also be toxic to alga species, as the parent molecule.
The authors thank the management, IIBAT for providing necessary facilities to conduct the experiment.