ISSN: 2347-7830
ISSN: 2347-7830
Abdolsamad S1*, Younes G2 and Yaghoobi MM1
1Graduate University of Advanced Technology, Kerman, Iran
2Professor in Pharmaceutical Biotechnology, Shiraz University of Medical Sciences, Iran
Received: 15/08/ 2015 Accepted: 22/09/ 2015 Published: 29/09/2015
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
Chlorophyll as a green pigment found in plants, algae and cyanobacteria, this biomolecule considered as the most important molecule in photosynthesis, which allows plants to absorb energy from light. In other hand β-carotene as a colored pigment with antioxidant activity currently is the most food additive. Beside one, metal nanoparticle [NPs], attracts an increasing interest, due to their new and different chemical, physical and biological characteristic as compared with those of macroscopic phase that allows attractive application in various fields. Chlorella vulgaris is a single-cell of green algae, which believe to have high potential in photosynthetic efficiency that could serve as food source. In the current study we have evaluated the arginine coated AgNPs and naked AgNPs in two independent groups on chlorella vulgaris chlorophyll and β-carotene content. The biologically synthesized AgNPs were provided and used in the concentration of 100, 200 and 400 μg/mL. Micro algae also cultured in BG-11 medium. Afterward the AgNPs were sonicated and added into Erle nmeyer which contains Chlorella vulgaris. The evaluation of chlorophyll and β-carotene content were done in the day 0, and 16. To get our favorable result the Camiel Eijckelhoff & Jan Dekker method were used, which is based on light absorption in 668.2 nm and 646.8 nm and 450 nm wavelength, by means of spectrophotometry device. The row results which have gained by spectrophotometry were analyzed by SPSS and Prism5 software. Our result shows that the effects of arginine coated AgNPs in the concentration of 100μg/mL has the most positive effect, and naked AgNPs in the concentration of 400μg/mL, most negative effect on chlorophyll content in chlorella vulgaris. The evaluation of β-carotene also have done by the same method in 450 nm wavelength, analyzed results on β-carotene content also shows the same results.
Chlorophyll, Β-Carotene, Agnps, Naked, Arginine coated, Chlorella vulgaris, Nanotechnology.
The application of nanoscale materials and structures, usually ranging from 1 to 100 nanometer [ nm], is an emerging area of nanoscale and nanotechnology. Nanomaterial may provide solutions to technological and enviro nmental challenges in the areas of solar energy conversion, catalysis, medicine, water treatment and agricultural application [1]. Nanomaterial often show unique and considerably changed physical, chemical and biological properties compared to their macro scaled counterparts [2]. AgNPs are the key multifunctional applied in many different fields of modern technology including enviro nmental remediation [3], the food industry [4], Owing to their high chemical reactivity and capability to act as electron donors to catalyze a wide variety of reactions [5], AgNPs have been applied to changes biomolecule content, such as chlorophyll and β-carotene in plants and algae. Silver NPs can produce various reactive oxygen species [6] that cause oxidative injury to cells via lipid peroxidation and oxidation of thiol groups on protein and DNA [7]. Paradoxically, Ag is also a toxic micronutrient for phytoplankton and also is required in some fundamental cellular functions [8]. Chlorella vulgaris is a spherical shape microalga in about 2 to 10 μm in diameter [9], and has no flagella [10]. This microalga contains green photosynthetic pigment chlorophyll A and B in its chloroplast. Live cells due to their inter cellular and intra cellular activity, are exposed to free radicals. These free radicals due to have free electron in their valance layer causes unwanted reaction in the cells, which can cause death of cell and may be organism [11]. To capture this free radical the live cells need to apply antioxidant compound [12]. Chlorophyll [13] and β-carotene [14] are two important biomolecule that have high antioxidant activity, which encourage us to use this biomolecules as natural and green antioxidant [14].
Since we are looking to get the safest and cheapest source of demanded materials, green synthesis of such a pharmacy will be attract high interest. In the early studies on microalga and drug investigation, the e antioxidant property of Chlorella vulgaris were searched in 1985 and the result showed that this microalga has anti-tumor activity. Chlorellin is the active ingredient of cited microalga, which has proven its potential as natural antioxidant [15].
In the current study, firstly the microalga sample was providing from biotechnological department of Pharmacy School of Shiraz University of medical sciences. The Chlorella vulgaris cell line were cultured in BG-11 medium [6], in 250 mL Erle nmeyer flasks and stored in ambient temperature. To keep homogeneity of medium the flasks putted on electron power shaker. In the next step the biologically synthesized AgNPs were providing from pharmaceutics Department of Pharmacy School of Shiraz University of Medical Sciences, in two different groups, 1st group was naked AgNPs and 2nd group contains AgNPs, which coated by the Arginine amino acid. To add AgNPs into flasks the NPs should undergo sonication, so the defined measure of each AgNPs were weighted by laboratory scale and were inserted into independent falcons. Five mL of distilled water were added to each falcon and got ready to sonication and dispersion. After sonication dispersed AgNPs in defined amount were injected into separate flasks which contains microalga. The defined concentrations to do study are as: 100, 200 and 400 μg/mL.
This study has done triplicate and the aim of this research is to determine the concentration of chlorophyll α and β-carotene from microalga suspension .In any repeat, preparation for read adsorption should done, in current study we have used Camiel Eijckelhoff and Jan Dekker method [16], to measure the chlorophyll content, OD should measure in two 668.2 nm and 646.8 nm wavelengths [17], and to measure β-carotene content, OD should measure in 450 nm.
This method is known as acetone 80% [18], because the microalga cell suspension before transfer to spectrophotometry is solved in acetone 80%. As the samples made ready, 3 mL of cell suspension were harvested and injected to centrifuge specialized falcons and centrifuged within 10 min in 2500 rpm. In next step supernatant have been thrown out and 3mL of acetone 80% were added to remaining cell suspension. For 2nd time the centrifuge has done within 10 minutes, now the above phase of solution contains chlorophyll. Now 1 mL of this suspension was transferred to quartz cuvette by the sampler, and by distilled water the final volume reached to 2 mL. As final step the spectrophotometry device have been set up in double wavelengths 668.2 nm and 646.8 nm, then any time one of samples were injected into quartz cuvette, measurement have done in two described wavelengths, and total chlorophyll a have calculated by using the referred formula.
Beta Carotene Measurement
The used method is known as n-Hexane method [19], because the main cell suspension soluble in this method is n-hexane. The procedure is as follow, 1 mL of microalga cell suspension were harvested and injected to centrifuge specialized falcons and centrifugation have done within 5 min in 3000 rpm, then supernatant have been thrown out and 3 mL of ethanol and n-hexane in the ratio 2:1 were added to sediment cell suspension and then this solution have undergone vortex for 1 to 1.5 minutes. In the 2nd step, 2 mL of distilled water and 4 mL of n-hexane have added immediately at same time, and solution have undergone shaking and vortex again. Afterward centrifugation has done within 5 min in 3000 rpm. Now in the falcons is seen three separate phase, which the top phase is contains carotenoid compound, such β-carotene. As the final step in measurement of β-carotene we have harvested 1mL of the above phase, and injected into quartz cuvette. To read the absorption by the spectrophotometry be possible the final volume of liquid that should be in cuvette is 2mL, so we have added 1mL of distillated water into cuvette and put it to designed place in spectrophotometry, after set us and adjustment the wavelength has read in 450 nm and the row result were saved.
The descriptive statistical analysis of row data, which has been showed in the Tables 1 and 2, showed that generally the positive effect of Arginine coated AgNPs is more considerable than naked AgNPs. Response of chlorella vulgaris to AgNPs showed that this nanoparticles between day 0 and 16 in different and independent concentration, shows different effects on chlorophyll and β-carotene content. As showed in Table 1 the total chlorophyll content on Chlorella vulgaris in the presence of AgNPs has the most increasing effects in 100μg/mL of arginine coated Iron nanoparticles. The chlorophyll content is calculated using below formula:
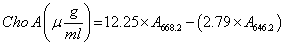
| AgNps(µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Time | 100 | 200 | 400 | 100n* | 200n* | 400n* |
| 1 | 0.09 ± 0.12 | 0.68 ± 0.14 | 0.63 ± 0.15 | 0.09 ± 0.11 | 0.10 ± 0.10 | 0.04 ± 0.08 |
| 16 | 4.51 ± 0.11 | 1.39 ± 0.09 | 0.01 ± 0.03 | 2.16 ± 0.12 | 0.47 ± 0.19 | 0.24 ± 0.11 |
Value are means± SE, n=2. In each row, significant differences at p<0.05 are shown
*; naked AgNPs
Table 1: Effects of AgNPs on chlorophyll content (µg/mL).
| AgNps(µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Time | 100 | 200 | 400 | 100n* | 200n* | 400n* |
| 1 | 1.05 ± 0.14 | 2.53 ± 0.20 | 3.69 ± 0.14 | 4.47 ± 0.13 | 5.64 ± 0.11 | 5.72 ± 0.20 |
| 16 | 4.23 ± 0.15 | 4.28 ± 0.14 | 4.23 ± 0.11 | 5.39 ± 0.16 | 6.39 ± 0.14 | 3.38 ± 0.20 |
Value are means± SE, n=2. In each row, significant differences at p<0.05are shown
*; naked silver AgNPs.
Table 2: Effects of AgNPs on ß-carotene content (µg/ml).
Chlorophyll content in the control samples gradually increase as same as in the other concentration treatments, but after passing days of experiment the chlorophyll content has changed. Our second measurement that was our final, on the day 16, the chlorophyll content in the 100μg/mL concentration of arginine coated AgNPs were significantly [p<0.05] differ from control Figure 1, in naked AgNPs Figure 2 as same as arginine coated AgNPs, the chlorophyll content in the concentration of 100μg/mL was significantly [p<0.05] differ from control sample. Paradoxically in the concentration of 400 μg/mL of arginine coated AgNPs the most decreasing effects were observed. Such a decrease in the concentration 400 μg/mL of naked AgNPs was observed, but the decrease was in the lowest range than arginine coated AgNPs.
All information that we have discussed above are shown in the Figure 1. As it has shown in the Figure 1 the comparison between all 3 samples and control sample, is clear that the most increasing effects of AgNPs on the chlorophyll content on Chlorella vulgaris microalga, is in the 100μg/mL concentration. Paradoxically decreasing effects is in the concentration 400 μg/ mL of AgNPs.
Compared to chlorophyll, than carotenoids, especially, β-carotene was less susceptible to Iron nanoparticles [AgNPs]. The information which has showed Table 2 the less susceptibility of β-carotene is proven.
Based on our analysis which have calculated on the formula [2], in all treatments, exception 400n after passing 16 days, increasing in the β-carotene was significantly differ from control [p<0.05]. The result shows that naked AgNPs is significantly toxic to Chlorella vulgaris cells higher than arginine coated AgNPs. The comparison is also drawn in the Figure 3.
As showed in the Figure 3 increasing in the β-carotene content after 16 days in all samples is near to each other except in the 200μg/mL that showed the highest increase in the β-carotene content. In other hand the most decrease in the β-carotene content is observed in the 400μg/mL sample, in naked AgNPs.
Metal nanoparticles in high concentration will be toxic to live cells, our finding in the current study also prove it. As we have shown in the 400μg/mL of AgNPs concentration toxicity was high and cause death in microalga cells [7]. In other hand the differences between toxicity of naked AgNPs and arginine coated [20]. AgNPs is also considerable. Since the naked AgNPs can more easily release their valance layer electrons, so in the high concentration valance layer electrons comes in rapid reaction with other available electrons in the media, it cause a lot unwanted and undesirable reaction, which cause the cell to undergo stresses and sometimes dead.