ISSN: 2322-0066
ISSN: 2322-0066
Juan Jose Montoya1,1a , Youness Ouahid1,1b, Carbache MF1, Eugenio Rodriguez Nunez1, Empar Sainz1, Antoni Molera1, Torres CF5, Reglero G5,6, Joaquin Carballido Rodríguez2,3, Castan Pablo1,4*
1 MiRNAX Biosens Research & Development Unit (MBR&DU). Avda. Industria, nº 4, building 1, ground floor, premises D (Natea Business Park) - ALCOBENDAS (Madrid), Spain
1a Department of Medicine, Universidad Complutense de Madrid, 28040 Madrid, Spain
1b Chronic Research & Development Unit (MBR&DU). Avda. Industria, nº 4, building 1, ground floor, premises D (Natea Business Park) - ALCOBENDAS (Madrid), Spain
2 Urology Service of the Puerta De Hierro University Hospital. C. Joaquín Rodrigo, 1, 28222 Majadahonda, Madrid, Spain
3 Department of Medicine, Autonomous University of Madrid (UAM). C. Arzobispo Morcillo, 4, 28029 Madrid, Spain
4 Hospital Carlos III de Madrid (HCIII-ISCIII) in collaboration with MBR&DU. C. de Sinesio Delgado, 10, 28029 Madrid, Spain
5 Institute of Food Sciences Research CIAL CSIC-UAM. Nicolas Cabrera 9, Madrid 28049, Spain
6 Production and Development of Foods for Health, IMDEA Food Institute, CEI UAM CSIC, Carretera de Canto Blanco 8, Madrid 28049, Spain
Received: 01-Jun-2024, Manuscript No. JOB-24-137859; Editor assigned: 05-Jun-2024, PreQC No. JOB-24-137859 (PQ); Reviewed: 19-Jun-2024, QC No. JOB-24-137859; Revised: 26-Jun-2024, Manuscript No. JOB-24-137859 (R); Published: 03-Jul-2024, DOI: 10.4172/2322- 0066.12.2.006.
Citation: Pablo C, et al. Immune Rousing and Inflammatory Modulation as Key Bottlenecks for Improving Cancer Outcome; The use of Bioactive Compounds from Rosmarinus Officinalis as Therapy Boosters.RRJ Biol. 2024; 12:006.
Copyright: © 2024 Montoya JJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews: Research Journal of Biology
Animal studies provide evidences of changes in tumour growth and cancer progression when subjects are fed with diets including certain bioactive elements. Unluckily, the molecular mechanisms through which most diet-derived ingredients contribute to improve cancer response are still to be characterized. In this context, the application of certain “omics” techniques such as, transcriptomics, proteomics, metabolomics and metagenomics have pointed at general mechanisms positively affected by precision nutrition in terms of modulating the immune system's responses to harmful stimuli, such as damaged cells, toxic compounds or uncontrolled cell growth. Those mechanisms normally correlate with a) the modulation of the inflammation needed to remove injurious stimuli triggering the healing process and b) the stimulation of the immune pliability needed to expand antitumor responses. Interestingly enough, increased immune capabilities and reduced inflammatory responses have also been historically observed in individuals with healthy life-styles and nutritional habits.
This work reports the use of one advanced machine learning model used to analyse modelled transcriptomes and proteomes from 7.500 oncologic patients in 3 major cohorts including Lung Cancer (LC), Colorectal Cancer (CRC) and Breast Cancer (BC). The analysis aimed to gain molecular knowledge on the two aforementioned mechanisms through which bioactive compounds induce the activation of the immune system and reduce the inflammatory response associated to cancer related harmful stimuli. Cross-sectional analysis and scrutiny between a) the Molecular Trigger Points (MTPs) elucidated by the A.I. and b) the reported mechanism of action for extracts from Rosmarinus officinalis L. (syn Salvia Rosmarinus Spenn.) suggest fitness for use of a bioactive formula based on diterpenic phenols from rosemary, formulated with bioactive alkylglycerols (OncoLipchronic® WO/2017/187000) in cancer patients. Furthermore, the high correlation between the MTPs and the effects registered for the bioactive formula open the space for further clinical confirmation of the immune activation and inflammatory modulation in cancer patients in order to define if a better clinical outcome can be obtained associating the purely medical treatment to the supplementation with OncoLipchronic® (Lipchronic).
Precision nutrition; Cancer; Bioactive compounds; Inflammatory and Immune modulation
As a major cause of death worldwide, 8.2 million deaths are directly attributed to cancer and such number is only bound to increase as a results of the global aging [1]. According to the accepted classification into two categories, metastatic cancer is the primary cause of cancer-related deaths, while non-metastatic cancer is less lethal [2]. Metastasis can occur both in the later stages of cancer progression and/or during the early stages of tumor development [3]. During metastasis, disseminating cancer cells escape from the status defined as oncologic equilibrium in which the tumour and immune system are in a dynamic balance where anti-tumour immunity controls cell growth, but is unable eliminate the tumour and tumour cells appear as clinically dormant [4,5]. The point at which the equilibrium is lost largely depends on the array of interactions between the neoplastic cells and the Extracellular Matrix (ECM) which embeds three elements of immune modulation: The mesenchymal support cells, the endothelial cells, and the infiltrated inflammatory immune cells. Cross-talk between these elements had been difficult to fully map until new "omics" techniques, such as transcriptomics, proteomics, metabolomics, and metagenomics, revealed the pathways leading to the deterioration of healthy tissue architecture and the development of an unhealthy microenvironment characterized by a compromised ECM and chronic inflammation [6]. Particularly, the presence of cancer-associated inflammation along the stages of tumorigenesis, contributes to genetic instability, epigenetic modification and induction of cancer proliferation. Likewise, chemical imbalance of the EMC will hamper the immune modulation formerly present during the equilibrium causing the decrease of cancer anti-apoptotic pathways, stimulating the angiogenesis and ultimately boosting cancer dissemination [7].
Interestingly, transcriptomic and proteomic studies have not only helped to map the interactions mentioned earlier but also suggested that precision nutrition could be a powerful tool for modulating the immune system's responses to harmful stimuli. This approach positively impacts homeostasis by directly reducing inflammation and enhancing immune system function, contributing to maintaining equilibrium. Additionally, it influences immune responses to certain anticancer treatments [8]. In this sense, the proteomics and metabolomics consistently point at the mechanisms activated by increases in the concentration of Reactive Oxygen Species (ROS)as a one of the two key switches regulating the dynamic interaction between immune system and tumour cells. Moreover, the upsurge of ROS has been confirmed by the aforementioned techniques to correlate with different epigenetic changes in the promoter region of tumour suppressor genes, leading to metastasis [9]. The second trigger point for tumour progression reported by such techniques correlates with systemic immune inflammation, a condition accompanying the upsurge of ROS whose effects manifest through parallel increase in the number of inflammatory cells (neutrophils, lymphocytes, monocytes). Such increase can either affect individual cell lines or the combination of Neutrophils-Lymphocytes-Ratio (NLR) both worsening the Tumour Immune Microenvironment (TME) with a decrease of their functional effects as key mediators of the immune system [10].
Curiously enough, a number of publications have positioned certain types of chemical extracts from Rosemary, (Rosmarinus officinalis L. syn. Salvia rosmarinus) as a potential antitumor agent in two general pathways: A) the reduction of ROS levels through the modulation of lipid metabolism and b) the reduction of systemic inflammation through the increase of cytotoxic effectors liberated by lymphocytes [11,12]. Those specific chemical extracts from Rosemary are very rich in phenolic diterpenes receiving the name of Supercritical Extracts (SFREs) and have been recently approved for human consumption [11,12].Taking into account that the mechanisms of action reported for the Rosemary SFREs overlap the two trigger points identified by proteomics and metabolomics in the maintenance of the oncologic equilibrium where anti-tumour immunity controls cell growth, we used this information to train the second-generation advanced machine learning engine AI-scythech daresbury to look for correlations between the effects of standardized chemotherapeutics and Rosemary SFREs in terms of cancer progression. The AI executed a search over 7.500 [(2500-CRC) for colon cancer, (2500-BC) for breast cancer and (2500NSCLS) for non-small cell lung cancer] cases within a cancer-patient-model whose a) transcriptomes were modelled from NCBI’s gene expression Omnibus© and b) proteomes were modelled fromUniProt’s Proteome Database©. A deep AIdriven correlation analysis was specifically aimed to explore whether Rosemary SFREs synergised with chemotherapeutic and immune checkpoint inhibitors acknowledged to slow down cancer progression.
The second-generation advanced machine learning engine AI-scythech daresbury was trained to look for correlations between the effects of standardized chemotherapeutics and Rosemary SFREs over 7.500 [(2500-CRC) for colon cancer, (2500-BC) for breast cancer and (2500-NSCLS) for non-small cell lung cancer] cases within a cancer-patient-model of transcriptomes from NCBI’s Gene Expression Omnibus©. The training method was also used for model validation following the k-fold Cross Validation (k-fold CV) standard, aimed to reduce the over-fitting of the training with the same algorithm used in the subsequent analysis. To achieve this, a 3-fold CV was implemented splitting the whole dataset into 3 folds and training the AI 3 times. In each training, we iteratively left one different fold out for validation, and trained on the remaining 2 folds applied according to the three groups of modelled cancer-patient-transcriptomes including Lung Cancer (LC), Colorectal Cancer (CRC) and Breast Cancer (BC) patients. Although in normal practice a k=5 or 10 is used. The extreme amount of data for 7500 modelled cases forced a 3-fold CV (Figure 1).
After the AI training, an evaluation of correlation between the effects of standardized chemotherapeutics and Rosemary SFREs over 7.500 cases within a cancer-patient-model of proteomes modelled from UniProt’s Proteome Database© was executed, finding that the trained engine performed well on the unseen testing data and confirming both the low bias and low variance of the engine training and the model for analysis.
A free tutorial for readers to acquire hands-on visibility on the entire process of machine learning, the resulting set of algorithms used by the AI over the model and the toolkits for clinical prediction is available through the Breast Cancer Wisconsin (Diagnostic) Database. A cross entropy / log loss function was used in the machine learning and AI training as depicted in Table 1.
| Task | Error type | Loss function | Note |
|---|---|---|---|
| Regression | Mean-squared error | 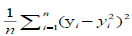 |
Easy to learn but sensitive to outliers (MSE, L2 loss) |
| Mean absolute error | 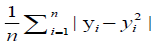 |
Robust to outliers but nut differentiable (MAE, L1 loss) | |
| Classification | Cross entropy =log loss | 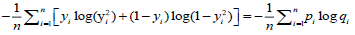 |
Quantify the difference between two probability distributions |
| Hinge loss | 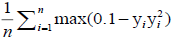 |
For support vector machine | |
| KL divergence | 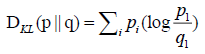 |
Quantify the difference between two probability distributions |
Table 1: Examples of commonly-used loss functions in machine learning. 7.500 [(2500-CRC) for colon cancer, (2500-BC) for breast cancer and (2500-NSCLS) for no small cell lung cancer] cases within a cancer-patient-model of transcriptomes were modelled from NCBI’s Gene Expression Omnibus© and crosschecked in overall input data with the reference databases listed in table 2.
The aforementioned design was implemented instead of a traditional ad-hoc healthcare data analytics to save the expert-intensive efforts needed to design hand-crafted features for collecting data from a limited unit of patients. In summary, the AI engine was successfully trained over the 7.500 transcriptomes modelled from NCBI’s Gene Expression Omnibus©, showing good performance on the unseen testing data for the cases included within the cancer-patient-model of 7.500 proteomes modelled from UniProt’s Proteome Database©. The cross entropy/log loss function was used confirming both the low bias and low variance of the engine training and the subsequent model for analysis, thus allowing the utilization of massive and complex Electronic Health Record (EHR) system data for the cancer-patient-model of 7.500 proteomes modelled from UniProt’s Proteome Database©.
After successfully training the second-generation advanced machine learning engine developed by AI-Scythech Daresbury following the 3-fold CV described, unseen data for the 7.500 cancer-patient proteomes modelled from UniProt’s Proteome Database© were scrutinised. Alteration patterns detected at proteomic level were re-confirmed over the 7.500 transcriptomes modelled from NCBI’s Gene Expression Omnibus© the AI engine was trained over. Re-analysis of the 7.500 transcriptomes [(2500-CRC) for colon cancer, (2500-BC) for breast cancer and (2500-NSCLS) for non-small cell lung cancer] confirmed the sustained pattern of activation of lipid metabolism and immune pathways accountable for systemic inflammation highlighted at proteomic level. Further confirmation was obtained by means of comparison to healthy proteomes obtained from the reference databases used for cross-checking of input data of transcriptomes modelled from NCBI’s Gene Expression Omnibus© (Table 2).
| Input Data | AI Model | Tumor | Function | Application | Ref*. |
|---|---|---|---|---|---|
| Spatial RNAseq data and images | DL: CNN | Breast cancer | Linking gene expression with visual features in cell morphology | ITH | [16] |
| ScRNA-seq data | DL: GAN, deep autoencoders | Colorectal cancer | Identifying the batchspecific cell types | ITH, TME | [12] |
| Bulk RNAseq and scRNA-seq data | ML: XGBoost | Lung adenocarcinoma | Predicting the prognosis of ligand– receptor gene pairs | TME | [13] |
Table 2: Reference databases used for cross-checking of input data of transcriptomes modelled from NCBI’s Gene Expression Omnibus© *Ref. numbers as published by Guy Y in 7.500 [(2500-CRC) for colon cancer, (2500-BC) for breast cancer and (2500-NSCLS) for non-small cell lung cancer] cases within a cancer-patient-model of proteomes were modelled from UniProt’s Proteome Database© using the same protected tool developed by AI-Scythech Daresbury that had been formerly used for the training and validation following the 3-fold CV described above.
Breaking down both general alterations accountable for systemic inflammation [(a) activation of lipid metabolism and (b) stimulation of immune pathways], the following operator were showing alterations across the three cancer-models (CRC, BC and NSCLS).
Curiously enough, the 6 operators confirmed to be induced across the 7.500 proteomes [(2500-CRC) for colon cancer, (2500-BC) for breast cancer and (2500-NSCLS) for non-small cell lung cancer] and re-confirmed at transcriptomic level by backward analysis of the transcriptomes the AI engine was trained over, converge in the activation of three key elements in the maintenance of the oncologic equilibrium. In short, the operators induced (Table 3) cause a downstream stimulation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a wide variety of Matrix Metalloproteinases (MMPs), and the Vascular Endothelial Growth Factor (VEGF); thus causing a loss of the dynamic balance where anti-tumour immunity controls cell growth.
| ROS-dependent operators induced | Colorectal Cancer (CR) | Lung Cancer (NSCLS) | Breast Cancer (BC) | Control (Healthy cohorts) |
|---|---|---|---|---|
| MAPK/ERK1/2 | Positively induced | Positively induced | Positively induced | No variation |
| MAPK/P38 | Positively induced | Positively induced | Positively induced | No variation |
| MAPK/C-Jun | Positively induced | Positively induced | Positively induced | No variation |
| N-terminal kinase (JNK) | Positively induced | Positively induced | Positively induced | No variation |
| Phosphoinositide-3kinase | Positively induced | Positively induced | Positively induced | No variation |
| Protein kinase B (PI3K/Akt). | Positively induced | Positively induced | Positively induced | No variation |
| Systemic Inflammation | INDUCED | INDUCED | INDUCED | UNDETECTED |
Table 3: ROS-dependent induction of a) Mitogen-activated protein kinase/extracellular signal regulated protein kinases 1/2 (MAPK/ERK1/2), b) Protein Kinases P38 and C-Jun, c) N-terminal Kinase (JNK), d) Phosphoinositide-3-kinase and e) Protein Kinase B (PI3K/Akt) detected in CR, NSCLS and BC proteomes also confirmed at transcriptomic level.
That force of positively retro-fed interactions between the neoplastic cells and the ECM directly leads of activation of the mesenchymal support cells, the endothelial cells, and the infiltrated inflammatory immune cells that has been widely reported to cause a to the loss of tissue architecture a corrupted ECM and chronic inflammation [6].
Over the pattern set by those findings, the most recent layer of reported activities for rosemary SFREs deserves analysis. As soon as rosemary SFREs were approved for human consumption, a series of scientific publications reported activities for such products rich in phenolic triterpenes, as a potential antitumor agent. More accurately, a new rosemary SFRE formulated with alkylglycerols to improve the bioavailability of the bioactive compounds called Lipchronic® has been patented (WO/2017/187000) and confirmed for safety of use. That particular product has been reported to have strong effects on the inhibition of lipid metabolism and on the reduction of systemic inflammation [11, 12]. Moreover, that new rosemary SFRE formulated with alkylglycerols has been postulated to synergize with chemotherapeutic and immune inhibitors in lung, breast and colon cancers [13-15]. Such information maps precisely with the data obtained through the AI analysis about the operators induced (Table 3 and Figure 2). In fact, the new Rosemary SFRE formulated with bioactive alkylglycerols, lipchronic® (WO/2017/187000), appears to reject the effects of the stimulation of the NF-KB, the MMPs, and the VEGF reported here by the second-generation advanced machine learning engine developed by AI-Scythech Daresbury.
Figure 2: Combined biomarker levels in Lung Cancer (NSCLS) modelled patients 1 (red), Colorectal Cancer (CR) modelled patients 2 (dark pink), Breast Cancer (BC) modelled patients 3 (light pink) and healthy controls cohorts (over 55 years’ old in light blue and below 54 years old in light blue). As described in Table 3 (a) Mitogen-activated protein kinase/extracellular signal-regulated protein kinases 1/2 (MAPK/ERK1/2), b) Protein Kinases P38 and C-Jun, c) N-terminal Kinase (JNK), d) Phosphoinositide-3-kinase and e) Protein kinase B (PI3K/Akt)) 2.500 patients each 1.250 patients each.
At this point, further clinical evidence and molecular characterisation of the pathways through which the new Rosemary SFRE formulated with bioactive alkylglycerols, Lipchronic® (WO/2017/187000) operates in relation to cytotoxic T lymphocytes and Effector Memory (EM) T lymphocytes may lead to fully understand if a noticeable reduction of systemic inflammation can be observed in cancer patients associated to the experimental treatment with Lipchronic®.
Predictions based on the data reported here by the second-generation advanced machine learning engine developed by AI-Scythech Daresbury suggest fitness for use of compounds capable of producing activation of the immune system and reduction of the inflammatory response associated to cancer-related harmful stimuli. In this sense, a cross-sectional analysis between the molecular MTPs elucidated by the AI. (Table 3 and Figure 2) and the reported mechanism of action the new bioactive formula based on diterpenic phenols from rosemary (Lipchronic® WO/2017/187000) in cancer patients, open the space for further clinical confirmation of immune activation and inflammatory modulation [16]. Should the effects described here as overlapping in cancer-modelled cohorts be confirmed through a canonical clinical trial in real patients, solid statements about better clinical outcomes could be made associating the purely medical treatment to the supplementation with Lipchronic®.
This work highlights the usefulness of AI-driven analysis instead of traditional ad-hoc healthcare data analytics, which usually requires expert efforts for designing and collecting data. Conclusions drew from the machine learning-based approach followed in this work have helped recognize patterns inside the data and allowed to perform personalized clinical predictions for lung, colon and breast cancer patients. Furthermore, the use of more generalizable models such as the ones described here across the 7.500 transcriptomes and proteomes [(2500-CRC) for colon cancer, (2500-BC) for breast cancer and (2500-NSCLS) for non-small cell lung cancer] have proven useful to maximize the utilization of massive but complex EHR data. On a more specific note, the usefulness of this AI-driven analysis has been proven by identifying the mechanisms through which a new Rosemary SFRE formulated with bioactive alkylglycerols, Lipchronic® (WO/2017/187000) should couple immune activation and inflammatory modulation in cancer patients for a better clinical outcome. Thus opening the space for further clinical trials associating the purely medical treatment to the supplementation with Lipchronic® in colon, lung and breast cancers.