ISSN: 2320-2459
ISSN: 2320-2459
S Saravanan1* S Rajesh2 and R Palani3
1Department of Engineering Physics (FEAT), Annamalai University, Annamalai Nagar-608002, Tamil Nadu, India
2Department of Physics, Annamalai University, Tamil Nadu, India
3Department of Physics, D.D.E, Annamalai University, Tamil Nadu, India
Received date: 01/02/2014; Revised date: 15/02/2014 Accepted date: 28/02/2014
Visit for more related articles at Research & Reviews: Journal of Pure and Applied Physics
Ultrasonic velocity, adiabatic compressibility, apparent molar compressibility, molar hydration number, and apparent molar volume.
Amino acids are important biologically active and basic structural units of proteins. However, due to the complex of conformation and configuration three dimensional structures of proteins. Amino acids and peptides have been taken up as modal compounds for understanding the behavior of more complex protein molecules in solutions. Amino acids in aqueous solution are ionized and can act as acids or bases. Knowledge of acids-base property of amino acids is extremely important in understanding many properties of proteins [1]. It is well-known that electrolytes influence the stability of proteins [2]. L-serine is a non-essential amino acids synthesized by hydrolysis of many proteins. L-serine plays an important role in the catalytic function of many enzymes. L-valine is an essential amino acid that is necessary for smooth nervous system and cognitive functioning. L-phenylalanine is an essential amino acid and is classified as non-polar, because of the hydrophobic nature of the benzyl side chain. Some studies have revealed that the presence of an electrolyte drastically affects the behavior of amino acids and peptides in solutions, and this fact can be used for their separation and purification. potassium nitrate is used as a diuretic in medicine. It also includes as an ingredient in toothpaste. It makes the toothless sensitive to pain. potassium nitrate affects nucleic acid synthesis in the greening cucumber cotyledons and the stability of tropomyosin [3]. In general the electrolytes present in our body influence the properties of biological molecules like proteins [4,5] which are a vital part of our body [6].
Ultrasonic velocity measurements have been successfully employed to detect and assess weak and strong molecular interactions [7]. The density, viscosity, ultrasonic velocity and its derived parameter are sensitive to structural changes that occur in solutions and to any interactions between solvent and solute [8,9]. The adiabatic compressibility studies of amino acids in salts solutions are few [10,11]. In this study, the ultrasonic velocity, density and viscosity values for amino acids: L-serine, L-valine, L-phenylalanine in aqueous and aqueous KNO3 solutions (0.5 and 1.0 mol.dm-3) at 308.15K, have been reported. Using these experimental values, the adiabatic compressibility, molar hydration number, apparent molar compressibility, apparent molar volume, limiting apparent molar compressibility, limiting apparent molar volume, and their constants (SK, Sv) transfer limiting apparent molar adiabatic compressibility, transfer limiting apparent molar volume, and viscosity A and B-Coefficients of Jones-Dole equation have been evaluated. These results have been rationalized in terms of the various interactions operating in these systems.
Analytical reagent (AR) and spectroscopic reagent (SR) grades with minimum assay of 99.9% of L-serine, Lvaline, L-phenylalanine and potassium nitrate are obtained from E-Merck, Germany and SdFine Chemicals, India, which are used as such without further purification. water used in the experiment was deionised, distilled and degassed prior to making solutions. Solutions of aqueous potassium nitrate (0.5 - 1.0 mol.dm-3) were prepared by volume and used on the day they were prepared. Solution of amino acids in the concentration range of 0.02-0.1 mol.dm-3 was made by mass on the molarity concentration scale with precision of ± 1 × 10-4g on an electronic digital balance (Model: SHIMADZU AX-200). The density was determined using a specific gravity bottle by relative measurement method with an accuracy of ± 0.01 kgm-3. An ultrasonic interferometer having the frequency of 2 MHz (MITTAL ENTERPRISES, New Delhi, Model: F-81) with an overall accuracy of ± 0.1% has been used for velocity measurement. An electronic digital operated constant temperature bath (Raaga Industries, Model: ULTRA COLD CHAMBER-437) has been used to circulate water through the double walled measuring cell made up of steel containing the experimental solution at the desired temperature. The accuracy in the temperature measurement is ± 0.1 K.
Using the measured data, the following volumetric, compressibility and transport parameter have been calculated using the standard relations.
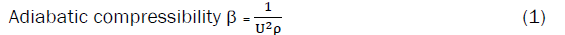
Molar hydration number has been computed using the relation

where, and βo are adiabatic compressibilities of solution and solvent respectively, n1 and n2 are number of moles of solvent and solute respectively.
The apparent molar compressibility has been calculated from relation.

where,β , ρ and β0, ρ0 are the adiabatic compressibility and density of solution and solvent, respectively, m is the molar concentration of the solute and M the molecular weight of the solute. φK is the function of m as obtained [12,13] and is given by

where,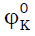 is the limiting apparent molar compressibility at infinite dilution and SK is a constant.
is the limiting apparent molar compressibility at infinite dilution and SK is a constant. 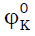 and SK of equation 4 have been evaluated by least square method.
and SK of equation 4 have been evaluated by least square method.
The apparent molar volume φV has been calculated using the relation.

The apparent molar volume φV has been found to differ with concentration according to [14] empirical relation as:

where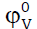 is the limiting apparent molar volume at infinite dilution and Sv is a constant and these values were determined by least square method.
is the limiting apparent molar volume at infinite dilution and Sv is a constant and these values were determined by least square method.
Transfer volumes, and transfer adiabatic compressibilities of each amino acid, 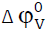 and
and 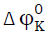 from water to aqueous potassium nitrate solutions were calculated using the equation.
from water to aqueous potassium nitrate solutions were calculated using the equation.
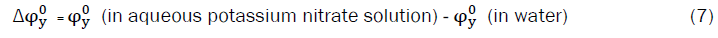
where, 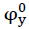 denotes limiting apparent molar volume
denotes limiting apparent molar volume 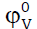 , and limiting apparent molar adiabatic compressibility
, and limiting apparent molar adiabatic compressibility 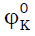
The importance of viscometric study of electrolyte solution in mixed solvent systems is well established [15,16]. The entire viscosity data have been analyzed in the light of Jones-Dole semi empirical equation [17].

where, η and ηo are the viscosities of the solution and solvent respectively and m are the molar concentration of the solute. A and B are constants which are definite for a solute-solvent system. A is known as the Falkenhagen coefficient which characterizes the ionic interaction and B is the Jones-Dole or Viscosity B-coefficient which depends on the size of the solute and the nature of solute-solvent interactions.
The experimental values of density (ρ), viscosity (η), and ultrasonic velocity (U), for different molar composition of each of the three amino acids viz., L-serine, L-valine and L-phenylalanine in aqueous potassium nitrate solutions are shown in Table-1. The values of adiabatic compressibility (β), molar hydration number (nH), apparent molar compressibility ( φK ), apparent molar volume ( φV ), limiting apparent molar compressibility (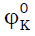 ), limiting apparent molar volume (
), limiting apparent molar volume (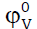 ), and their constants (SK, Sv), transfer adiabatic compressibility (
), and their constants (SK, Sv), transfer adiabatic compressibility (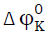 ), transfer volume (
), transfer volume (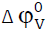 ), and viscosity A and B coefficient of Jones-Dole equation are calculated and the results are given Tables 2-3. Further, the Figs.1-2 show the variation of transfer adiabatic compressibility and transfer volume of L-serine, L-valine, and L-phenylalanine in aqueous potassium nitrate solutions at 308.15 K and the curves are drawn using least square fitting.
), and viscosity A and B coefficient of Jones-Dole equation are calculated and the results are given Tables 2-3. Further, the Figs.1-2 show the variation of transfer adiabatic compressibility and transfer volume of L-serine, L-valine, and L-phenylalanine in aqueous potassium nitrate solutions at 308.15 K and the curves are drawn using least square fitting.
Table 3:Values of limiting apparent molar compressibility ( 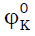 ), limiting apparent molar volume (
), limiting apparent molar volume ( 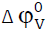 ), and their constants SK and Sv, transfer adiabatic compressibility (
), and their constants SK and Sv, transfer adiabatic compressibility (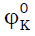 ), transfer volume (
), transfer volume (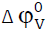 ), and A and B co- efficient of Jones-Dole equation of the amino acids in aqueous potassium nitrate solutions at 308.15 K for
), and A and B co- efficient of Jones-Dole equation of the amino acids in aqueous potassium nitrate solutions at 308.15 K for
Figure 2: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
In all the three amino acids systems (Table-1), the values of density and ultrasonic velocity increase with increase in molar concentration of amino acids as well as potassium nitrate (KNO3) content. This increasing trend suggests a moderate strong electrolytic nature in which the solutes (amino acids) tend to attract the solvent (aqueous potassium nitrate) molecules. Molecular interaction is thus responsible for the observed increase in density and ultrasonic velocity in these mixtures. The factors apparently responsible for such behavior may be due to the presence of interactions caused by the proton transfer reactions of amino acids in aqueous potassium nitrate mixtures. The increase in the ultrasonic velocity in these solutions may be attributed to the cohesion brought about by the ionic hydration [18]. In all the three systems, the value of adiabatic compressibility (Table-2) decreases with increase in concentration of solute (amino acids) as well as increase in concentration of aqueous KNO3. The decrease in adiabatic compressibility is attributed to the influence of the electrostatic field of ions (NH3+ and COO-) on the surrounding solvent molecules (K+,NO3 -) called electrostriction. The magnitudes of β values are larger in L-serine than in other two amino acids. The larger β value which shows molecular associations / interactions is greater in L-serine than in two amino acids. Amino acids molecules in the neutral solution exist in the dipolar form and thus have stronger interaction with the surrounding water molecules. The increasing electrostrictive compression of water around the molecules results in a large decrease in the compressibility of the solutions. This result in the present study generally confirms the conclusions drawn earlier from the velocity data. The interaction between the solute and the water molecules present in the solvent can be termed as hydration. From (Table-2) it is observed that the positive values of nH indicate an appreciable solvation of solutes [19]. This is an added support not only for the structure promoting tendency of the solutes but also for the presence of appreciable dipole-dipole interactions between solute and water molecules. This also leads to further suggestion that the compressibility of the solution will be less than that of the solvent. As a result, solutes will gain mobility and hence there will be more probability of conducting solvent molecules. This may further enhance the interaction between solute and solvent molecules. The values of nH decrease with increase in concentration of amino acids in all systems. But these values are found to increase with increasing the concentration of potassium nitrate solutions. The decreasing behavior of nH shows that the strength of interaction gets weakened between the ion-solvent molecules, but however it increases the ion-ion interaction in the mixtures [20].
The following observation has been made on φK and φV (Table-2) of the three amino acids in aqueous potassium nitrate solutions at 308.15 K.
(i) The values of φK and φV are all negative over the entire range of the molarity.
(ii) The negative values of φK and φV increase with the increase in concentration of amino acids, but it is found to decrease with increasing the contents of KNO3.
(iii) The magnitude of φK is in the order: L-serine > L-valine > L-phenylalanine.
The above observation clearly suggests that the negative values of φK and φV in all systems indicate the presence of ion-solvent interactions. Since more number of water molecules is available at lower concentration of potassium nitrate, the chances for the penetration of solute molecules into the solvent molecules are highly favored. The decrease in φV is due to strong ion-ion interaction and vice-versa. The observed behavior of φK reveals the strengthening of the ion-solvent interaction in all systems studied. The negative values of φV indicate electrostrictive solvation of ions [21]. From the magnitude of φK , it can be concluded that stronger molecular association is found in L-serine than in other two amino acids and hence L-serine is a more effective structure maker.
The limiting apparent molar compressibility 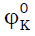 provides information regarding ion-solvent interaction and SK, that of ion-ion interactions in the solution. From (Table-3) it is observed that
provides information regarding ion-solvent interaction and SK, that of ion-ion interactions in the solution. From (Table-3) it is observed that 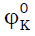 values are negative and they increase with increasing the concentration of potassium nitrate in all systems studied. Appreciable negative values of
values are negative and they increase with increasing the concentration of potassium nitrate in all systems studied. Appreciable negative values of 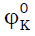 and its behavior for all systems reinforce our earlier view regarding the existence of ion-solvent interaction in the mixtures. The negative values of
and its behavior for all systems reinforce our earlier view regarding the existence of ion-solvent interaction in the mixtures. The negative values of 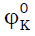 (loss of compressibility of the medium) indicate that the water molecules surrounding the amino acids molecules present a greater resistance to compression than the bulk [22]. The magnitude of
(loss of compressibility of the medium) indicate that the water molecules surrounding the amino acids molecules present a greater resistance to compression than the bulk [22]. The magnitude of 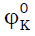 is in the order L-serine > L-valine > L-phenylalanine. The values of SK exhibit positive value and they decrease with increasing the concentration of potassium nitrate in all the three amino acids. This behavior indicates the existence of ion-ion interaction with increase in potassium nitrate content and suggests structure making / breaking effect of the amino acids. It is well known that solutes causing electrostriction lead to decrease in the compressibility of the solution. This is reflected by the negative values of φK of the amino acids. The volume behavior of a solute at infinite dilution is satisfactorily represented by
is in the order L-serine > L-valine > L-phenylalanine. The values of SK exhibit positive value and they decrease with increasing the concentration of potassium nitrate in all the three amino acids. This behavior indicates the existence of ion-ion interaction with increase in potassium nitrate content and suggests structure making / breaking effect of the amino acids. It is well known that solutes causing electrostriction lead to decrease in the compressibility of the solution. This is reflected by the negative values of φK of the amino acids. The volume behavior of a solute at infinite dilution is satisfactorily represented by 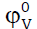 which is independent of the ion-ion interactions and provides information concerning ion-solvent interactions. (Table-3) reveals that the values of
which is independent of the ion-ion interactions and provides information concerning ion-solvent interactions. (Table-3) reveals that the values of 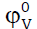 are negative in all the three studied amino acids. The values of
are negative in all the three studied amino acids. The values of 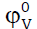 increase with the addition of potassium nitrate contents in all the systems studied. The increase in
increase with the addition of potassium nitrate contents in all the systems studied. The increase in 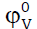 may be attributed to the increased hydrophilicity / polar character of the side chain of the amino acids. It is evident from the Table -3 that Sv is positive in all the three systems suggesting the presence of strong ion-ion interactions.
may be attributed to the increased hydrophilicity / polar character of the side chain of the amino acids. It is evident from the Table -3 that Sv is positive in all the three systems suggesting the presence of strong ion-ion interactions.
The values of transfer adiabatic compressibility 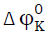 and transfer volume
and transfer volume 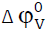 (Table-3) are positive and they increase with increase in the concentration of potassium nitrate in all the three amino acid systems which suggest the existence of strong ion-solvent interactions in the mixtures. Generally, the interaction between amino acids and potassium nitrate can be classified as: (i) ion-ion interaction among the K+, and NO3- ions and (COO-,NH3+) zwitterionic end groups, (ii) ion-hydrophilic interactions between ions and hydrophilic groups (-CONH2,-CONH) of amino acids, (iii) ion-nonpolar group interaction occurring between ions and the nonpolar groups (-CH2/-CH3) of amino acids. The
(Table-3) are positive and they increase with increase in the concentration of potassium nitrate in all the three amino acid systems which suggest the existence of strong ion-solvent interactions in the mixtures. Generally, the interaction between amino acids and potassium nitrate can be classified as: (i) ion-ion interaction among the K+, and NO3- ions and (COO-,NH3+) zwitterionic end groups, (ii) ion-hydrophilic interactions between ions and hydrophilic groups (-CONH2,-CONH) of amino acids, (iii) ion-nonpolar group interaction occurring between ions and the nonpolar groups (-CH2/-CH3) of amino acids. The 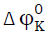 and
and 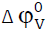 values can also be explained on the basis of co-sphere overlap model [23] in terms of solute-co-solute interactions. According to this model, ion-ion and ion-hydrophilic group interactions contribute positively, whereas ion-non-polar group interactions contribute negatively to the
values can also be explained on the basis of co-sphere overlap model [23] in terms of solute-co-solute interactions. According to this model, ion-ion and ion-hydrophilic group interactions contribute positively, whereas ion-non-polar group interactions contribute negatively to the 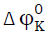 and
and 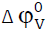 values. Therefore, from Figs. 1-2, the positive
values. Therefore, from Figs. 1-2, the positive 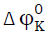 and
and 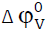 values observed in all the three amino acids suggest that the interaction contribution of type (i) and (ii) is much stronger than that of type (iii). The magnitude of
values observed in all the three amino acids suggest that the interaction contribution of type (i) and (ii) is much stronger than that of type (iii). The magnitude of 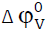 is in order: L-serine < L-valine < L-phenylalanine.
is in order: L-serine < L-valine < L-phenylalanine.
Viscosity is another important relation in understanding the structure as well as molecular interaction occurring in the mixtures. From Table-1 it is observed that the values of viscosity increase with increase in molar concentration of amino acids as well as potassium nitrate content. This increasing trend indicates the existence of ion-solvent interaction occurring in these systems. In order to shed more light on this, the role of viscosity coefficients has been obtained. From the Table-3 it is observed that the values of A coefficient are positive for all the systems indicating the presence of ion-ion interactions [24]. Further, the values of the B-coefficient are positive in all systems studied. B-coefficient is also known as measure of order and disorder introduced by the solute into the solvent. It is also a measure of ion-solvent interaction and relative size of the ion and solvent molecules. The behavior of B-coefficient in all the three systems suggests the existence of strong ion-solvent interaction [25]. The magnitude of B values is in the order L-serine > L-valine > L-phenylalanine. This conclusion is in excellent agreement with that drawn from φK and 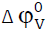 .
.
In the present work volumetric, compressibility and transport parameters of L-serine, L-valine and Lphenylalanine in aqueous potassium nitrate solutions at 308.15 K were obtained using density, viscosity and ultrasonic velocity data and the results have been used to study the existence of ion-solvent interactions. From the magnitude of φK , 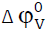 and the values of B-coefficient, it can be concluded that L-serine possesses strong ionsolvent interaction than the other two amino acids. The transfer adiabatic compressibility
and the values of B-coefficient, it can be concluded that L-serine possesses strong ionsolvent interaction than the other two amino acids. The transfer adiabatic compressibility 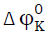 and transfer volume
and transfer volume 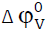 data suggest that ion-ion and ion-hydrophilic group interactions are dominating over the ion-non polar group interactions.
data suggest that ion-ion and ion-hydrophilic group interactions are dominating over the ion-non polar group interactions.