ISSN: 2322-0066
ISSN: 2322-0066
Ranjan Ramasamy*
ID-FISH and IGeneX, 556 Gibraltar Drive, Milpitas, CA 95035, USA
Received: 13-Feb-2024, Manuscript No. JOB-24-127453; Editor assigned: 19-Feb-2024, PreQC No. JOB-24-127453 (PQ); Reviewed: 04-Mar-2024, QC No. JOB-24-127453; Revised: 11-Mar -2024, Manuscript No. JOB-24-127453(R); Published: 18-Mar-2024, DOI: 10.4172/2322-0066.12.1.002.
Citation: Ramasamy R. Societal Factors Constraining the Application of Advances in Biological Sciences for Controlling COVID-19 and Mosquito and Tick-Borne Diseases. RRJ Biol. 2024;12:002.
Copyright: © 2024 Ramasamy R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews: Research Journal of Biology
While many advances in biological sciences have been applied in medicine, agriculture and industry with great benefit, some recent trends in society, governance structures and communications, appear to be hampering the use of new scientific findings for controlling infectious diseases. These limitations are illustrated with examples related to (i) the adaptation of fresh water mosquito vectors of major arboviral diseases to salinity in coastal areas with consequences for disease transmission, (ii) under-standing the implications of reduced dengue transmission during the COVID-19 lockdown for dengue control, (iii) causes un-derlying the rapid spread of the malaria vector Anopheles stephensi in South Asia and Africa, (iv) the application of serodiagnostic techniques for Lyme disease and tick-borne relapsing fever caused by tick-borne bacteria of the genus Borrelia, and (v) COVID-19 prevention.
Aedes aegypti; Aedes albopictus; Anopheles culicifacies; Anopheles stephensi; Arboviral diseases; Borreliosis; Dengue; COVID-19; Lyme disease; Malaria; Salinity adaptation in mosquito vectors; Sea-level rise; Serodiagnosis; Tick-borne relapsing fever; Vaccines
AFM: Atomic Force Microscopy; CDC–US: Centres for Disease Control and Prevention; COVID-19: Coronavirus Infectious Disease 2019; EIA: Enzyme Immunoassay; IFA: Immunofluorescence Assay; L1-L4: First To Fourth Instar Larval Stages; LC50: Concentration Producing 50% Lethality; LD: Lyme Disease; LDB: Lyme Disease Borreliae; MTTT: Modified Two-Tier Test; RFB: Relapsing Fever Borreliae; TBRF: Tick-borne Relapsing Fever; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SEM: Scanning Electron Microscopy; STTT: Standard Two-Tier Test; TEM: Transmission Electron Microscopy; WB: Western Blot; WHO: World Health Organization
The use of other living organisms and their products for human benefit has a very ancient origin. Identification of herbal medicines, selection of wild cereal crops to form cultivated varieties, domestication of goats, sheep and cattle, cultivation of cotton, and fermentation of plant products to form alcoholic beverages are some examples [1]. However, elucidation of the structure of DNA and proteins in the middle of the 20th century spawned the science of molecular biology which has been applied in innovative ways in medicine, agriculture, and industrial production for human benefit. For example, biotechnology, defined by the European Federation of Biotechnology in 1981 as ‘the integrated use of biochemistry, microbiology and chemical engineering in order to achieve the technological application of the capacities of microbes and cultured cells’, has created useful products such as monoclonal antibodies for clinical therapy of COVID-19, reagents for the laboratory diagnosis of infectious diseases [2-4], and the CRISPR-Cas9 gene editing technology for advancing human medicine, agriculture and animal husbandry [5].
Some societal limitations that presently have an impact on human well-being are well known. Two prominent examples are the (i) rapid expansion of human populations and improvements in their quality of life, largely attributable to science-based improvements in healthcare and food production, but leading also to the unsustainable use of the planet’s biotic and abiotic resources, and (ii) continuing high levels of greenhouse gas emissions and environmental pollution, causing many undesirable effects, including global warming and rising sea levels. While these two factors also influence the prevalence of infectious diseases, less-well understood societal factors that limit the application of recent advances in biological sciences for controlling infectious diseases have emerged. These constraints are discussed with specific examples from (i) the transmission of dengue by Aedes mosquitoes, (ii) the transmission of malaria by Anopheles culicifacies and Anopheles stephensi mosquitoes (iii) the application of serological techniques for the laboratory diagnosis of Lyme disease and tick-borne relapsing fever borreliosis, (iv) understanding immunity to SARS-CoV-2 and the delivery of COVID-19 vaccines.
Biology of Aedes mosquito vectors and the control of dengue and other arboviral diseases
Aedes aegypti mosquitoes are the principal vectors of human arboviral diseases, including dengue, chikungunya, yellow fever and Zika, in tropical and sub-tropical countries [6-8]. Aedes albopictus is an important secondary arboviral vector that has recently expanded its range to several temperate zone countries, partly through developing diapausing eggs able to survive winters. Both Aedes species are widely regarded to lay eggs and undergo preimaginal development only in Fresh Water (FW) habitats containing <0.5 gL-1 salt, so that the present World Health Organization (WHO) [6,7] and US Centres for Disease Control and Prevention (CDC) [8] guidelines for larval source reduction through applying larvicides and elimination of preimaginal habitats are only directed towards fresh water habitats. Larval source reduction measures are particularly important worldwide for controlling Aedes vectors, compared to the spraying of adulticides indoors and the use of insecticide impregnated bed nets which are effective against Anopheles mosquito vectors of malaria. This is because the two Aedes species, in contrast to the Anopheles vectors, tend to blood feed on humans out-doors and during the day. Most countries follow WHO recommendations for controlling arboviral diseases, including dengue which is the most prevalent arboviral disease worldwide with 5.2 million annual cases reported to the WHO in 2019 but with a higher estimated incidence of 100 million-400 million cases worldwide [6,7]. The WHO recommends measures to minimize or eliminate fresh water collections where the Aedes vectors oviposit and undergo preimaginal development, the application of the organophosphate larvicide Temephos at concentrations up to 1 mgL-1 to fresh water storage containers which constitute important Aedes larval habitats in the tropics, and space spraying with adulticides to control dengue epidemics [7,9].
Implications of the adaptation of fresh water Aedes mosquito vectors to salinity in coastal areas
Aedes aegypti and Aedes albopictus were first reported to be capable of ovipositing and undergoing preimaginal development to adulthood in Brackish Water (BW) habitats of up to 15 gL-1 salt in the northern coastal peninsula of Jaffna in the island of Sri Lanka (Figure 1) with FW, BW and saline water defined as containing <0.5, 0.5-30 and >30 gL-1 salt, respectively [10-15].
Figure 1: (a) Map showing the location of Sri Lanka in relation to South India; (b) the relative locations of the Jaffna pen-insula, Mannar island and Jaffna city within Sri Lanka; (c) sites within Jaffna city where Anopheles stephensi (discussed in below) larvae were found. Reproduced with permission under the creative commons license from [16].
The preimaginal development of Aedes aegypti and Aedes albopictus in BW has since been reported from the coastal areas of other countries including Brunei Darussalam, the US, Brazil, Mexico, Indonesia and India [17-22]. Typical BW habitats of Aedes aegypti are BW accumulations in beach debris, fishing boats, and coastal wells, as well as discarded containers in swamps, stagnant surface drains, surface ground water and household containers in coastal areas [10-15,17-22]. Some BW collections where Aedes aegypti larvae were found in Jaffna city and the Jaffna peninsula are illustrated in Figure 2.
Figure 2: BW habitats of Aedes aegypti in the Jaffna peninsula. Photographs show the brackish water collections containing larvae in: (A,B):disused boats; (C,E): abandoned wells; (D,F): discarded containers in beach debris. Reproduced with permission under the creative commons license from [10].
BW-derived Aedes aegypti and Aedes albopictus larvae in the Jaffna peninsula were more salinity-tolerant, possessing higher LC50 for salt than the corresponding FW-derived larvae from the Sri Lankan mainland as shown in Figure 3 [10,15,23]. Aedes albopictus was observed to be more salinity tolerant than Aedes aegypti in both locations (Figure 3).
Figure 3: Effect salinity on Aedes aegypti and Aedes albopictus from (A) coastal Jaffna peninsula and (B) mainland Sri Lanka on the L1 and L3 larvae to adult transformation. L1 and L3 refer to first and third instar larvae respectively. Ppt–parts per thousand or gL-1. Reproduced with permission under the creative commons license from [10]. 
Further investigations on laboratory-maintained colonies demonstrated that, although they remained reproductively compatible, BW-adapted or salinity-tolerant Aedes aegypti differed from FW Aedes aegypti significantly in several important features summarized in Table 1. The observed structural and physiological differences are likely to underlie the greater salinity tolerance of BW Aedes aegypti.
| Characteristic | Differences | Cited references |
|---|---|---|
| LC50 for salt | Significantly higher LC50 for salt in BW Aedes aegypti for the L1 and L3 to adult transition which was an inheritable characteristic | [10,15,23] |
| Osmoregulatory anal papillae in L3 larvae | Significantly larger anal papillae in BW Aedes aegypti which was an inheritable characteristic | [24] |
| Gene expression in mid-stage L4 larvae | Marked differences, particularly in genes for cuticle proteins, and others associated with cuticle synthesis | [25] |
| Protein composition of L4 cuticles | Marked differences compatible with the gene expression data | [25] |
| Cuticle structure by TEM | Thicker cuticles in L4 larvae and adult abdomen with more prominent endocuticles and exocuticles in BW Ae. aegypti | [25] |
| Surfaces of shed L3 and L4 cuticles | More pronounced surface undulations in BW Aedes aegypti cuticles by AFM and SEM | [15] |
| Egg sizes | Significantly smaller eggs in BW Aedes aegypti | [15] |
| Surfaces of eggs by AFM and SEM | BW Aedes aegypti egg surfaces were significantly less elastic by AFM, with more undulating surfaces seen by AFM and SEM | [15] |
| Hatchability of eggs and preimaginal development to adults | Hatchability of eggs and preimaginal development to adults by FW Aedes aegypti is decreased in 10 gL-1 salt BW. These properties were maternally inherited in genetic crosses | [15] |
| Susceptibility of L3 and L4 to the common larvicide Temephos | BW Aedes aegypti were significantly more resistant to Temephos than FW Aedes aegypti | [15] |
Note: L1-L4: first to fourth instar larval stages, AFM: Atomic Force Microscopy, SEM: Scanning Electron Micros-Copy, TEM: Transmission Electron Microscopy, LC50: concentration producing 50% lethality.
It is hypothesized that the salinity-adaptive structural and molecular changes seen in Aedes aegypti will be paralleled in the secondary arboviral vector Aedes aegypti, as well as other FW mosquito vectors that have adapted to develop in coastal BW, e.g., the malaria vectors An. culicifacies and An. stephensi as discussed.
The euryhaline nature of salinity-tolerant Aedes vectors in coastal areas and their neglect in vector control programs, al-lows them to serve as arboviral reservoirs in coastal areas and function as bridging vectors for adjoining inland areas, thereby enhancing arboviral disease transmission and facilitating epidemics as previously described [26]. As a consequence, it is predicted that small islands and countries with long coastlines in relation to their total land area, e.g. Indonesia, Sri Lanka and the Caribbean islands, will be particularly prone to the enhanced transmission of arboviral diseases like dengue, chikungunya and Zika [27,28]. Moreover, it is expected that global warming leading to a rise in sea levels will increase ground water salinization in coastal areas, and further exacerbate arboviral disease transmission in this manner [29,30]. Therefore, it is important in coastal zones throughout the world to (i) extend larval source reduction efforts also to the BW habitats of Aedes vectors, and (ii) monitor the efficacy of widely-used larvicides such as Temephos in coastal areas because of the demonstrated diminished susceptibility of BW Aedes to Temephos [15]. Dengue, the most prevalent arboviral disease world, causes >100,000 cases annually in Sri Lanka. Despite overwhelming published evidence beginning with the first demonstration of salinity tolerant Aedes vectors in Sri Lanka in 2011 and subsequently in other countries [17-22], Sri Lanka’s Ministry of Health has been slow to target BW collections in coastal areas in its dengue control program. The WHO and CDC guidelines for controlling dengue surprisingly continue to only focus on FW habitats of the Aedes vectors [6-9]. Because the WHO guidelines serve as the model for many countries, their modification to additionally target BW habitats of the two vectors can be an important advance globally for the more effective control of dengue and other arboviral diseases. The documented preimaginal development of both Aedes aegypti and Ae. albopictus in BW habitats in coastal areas of the state of Florida [18] and Aedes aegypti in neighbouring Mexico, suggests that modifying the present CDC guidelines [8] to also target coastal BW habitats of the two vectors will likewise benefit the control of dengue and other arboviral diseases in the US.
Implications of reduced Aedes vector densities and dengue incidence during the COVID-19 lockdown
Public health measures that severely curtailed the movement of people to reduce COVID-19 transmission during the period from 2020 to 2022, termed lockdown, were initially expected to adversely affect mosquito control programs and increase the incidence of diseases like malaria and dengue in endemic countries [31]. A significant increase in the incidence and mortality from malaria during the COVID-19 lockdown was indeed reported in Zimbabwe [32]. A small in-crease in dengue incidence was also reported from Singapore [33], a country that employs stringent larval source reduction measures in both public places and residences to control dengue transmission.
However, the incidence of dengue incidence markedly decreased in all districts of Sri Lanka during the COVID-19 lock-down when compared with the five years immediately preceding the pandemic [34-37]. An 89% reduction in the number of predicted dengue cases during the most drastic lockdown period from March 2020 to April 2021 in the northern Jaffna district (Figure 4) was accompanied by an 89% fall in Aedes larvae collected from ovitraps in Jaffna city [34,35].
Figure 4: Numbers of actual and predicted dengue cases in the Jaffna district of Sri Lanka during COVID-19 movement restrictions. Severe restrictions on the movement of people were in place from 1 March 2020 to 30 April 2021 (period A) to lower COVID-19 transmission. These were variably eased and reimposed from 1 May 2021 until 22 November 2021 (period B), but removed altogether with the full opening of all schools from 22 November 2021 onward (period C). Re-produced with permission under the creative commons license from [35]. 

Dynamics of disease transmission by vector mosquitoes for a non-immune population is described by the Ross–MacDonald equation which can be usefully applied to dengue transmission in the present context [38]. This equation relates the number of secondary infections generated from a single infected person ( R0) to vector parameters as follows:
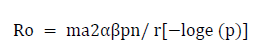
Where,
m=ratio of the number of vector mosquitoes to the number of humans;
a =average number of human blood meals taken by a mosquito in a day;
α=probability of transmission of pathogen from an infected human to a biting mosquito;
β=probability of transmission of a pathogen from an infected mosquito to a non-immune human during feeding;
p=daily probability of survival of the mosquito vector;
n=duration in days from infection of a biting mosquito until the mosquito becomes capable of infecting humans after the pathogen undergoes obligatory development in the mosquito, also termed the extrinsic incubation period;
r=recovery rate in humans (inverse of the average duration of infectiousness in days).
The anthropophagic Aedes aegypti and partly anthropophagic Aedes albopictus vectors are daytime feeders [6-9,39] that are highly prevalent in premises of schools, hospitals, government offices, transport hubs, and factories in Sri Lanka [14,40]. The closure of schools and offices, as well other forms of restrictions on the movement of people outside of their homes, can therefore be expected to reduce blood feeding by the two vectors, particularly Aedes aegypti, leading to a reduction in ‘a’ in the equation. Any reduction in blood meals will result in reduced oviposition and decreased vector densities, manifesting as reduced values of ‘m’ and ‘p’. Lower values for ‘a’, ‘m’ and ‘p’ will diminish R0, which will be particularly impacted by its exponential relationship with ‘a’ and ‘p’. R0 is directly related to the rate of dengue transmission and therefore the number of dengue cases in the population. Reduced oviposition as a result of decreased blood feeding is consistent with diminished larval collections from ovitraps in Jaffna city, which was also independently observed in the western Colombo district, during the lockdown in Sri Lanka
In particular, diminished access of Aedes vectors to blood meals in public places like schools, hospitals, government offices, transport hubs, factories and places of religious worship, during the COVID-19 lockdown can be postulated to re-duce vector densities and vector survival and therefore dengue transmission [34,35]. Additionally, infection of uninfected Aedes vectors through feeding on infected persons, and infection of uninfected persons by infected Aedes vectors in new locations are both promoted by movement of people, and could have contributed to reduced dengue incidence during the COVID-19 lockdown. Dengue and malaria incidences fell by 75% and 46% respectively during the 2020 lock-down in India, a country with similar dengue epidemiology and mosquito vector control programmers to Sri Lanka. Statistics for malaria were not available for Sri Lanka because malaria had been eliminated from the island in 2013 [41,42]. The early expectation of epidemiologists, and the WHO, that the incidence of dengue and malaria will increase due to a collapse of vector control programmers during the COVID-19 pandemic, was therefore not borne out in India and Sri Lanka. From the perspective of more effective dengue control in Sri Lanka, India and other countries with similar dengue epidemiology, it would appear that this prevalent view detracts from experimental findings which strongly suggest that dengue control measures involving Aedes vector larval source reduction measures need to be more stringently applied than at present in public places where people congregate.
It is suggested that greater awareness of the relevant scientific findings by pertinent national and international organizations and their adoption of appropriate measures can improve the control of dengue and other arboviral diseases. Beneficial measures can include promoting further epidemiological studies to confirm the importance of the reported findings and modifying influential national and international guidelines for the control of Aedes vectors.
Adaptation of fresh water Anopheles malaria vectors to salinity and urban water collections and the consequences for malaria control. The WHO in its latest World Malaria Report estimated 249 million cases of malaria occurred worldwide in 2022 (an increase of 5 million over 2021) and 608,000 deaths from malaria worldwide [43]. All known species of Plasmodium parasites that cause human malaria are transmitted by several different species of Anopheles mosquitoes [44].
The implications of freshwater Anopheles vectors adapting to salinity in coastal areas for malaria transmission
Some salinity-tolerant Anopheles that transmit malaria parasites in coastal areas have evolved into distinct species as a result of their reproductive isolation. These include an, farauti in Australia and neighbouring countries, An. sundaicus in Asia, An. melas and An. merus in Africa, as well as An. aquasalis and An. albimanus in the Americas [28-30]. It was recently observed for the first time that An. culicifacies, the principal rural FW vector of malaria in South Asia, can also lay eggs and undergo preimaginal development in BW of up to 4 gL-1 salt in coastal swamps of eastern Sri Lanka and the Jaffna peninsula [45,46]. The possibility that An. culicifacies may have similarly adapted to develop in coastal BW habitats of other South and Southeast Asian countries requires investigation.
Adaptation to salinity and man-made water sources, as well as societal conflicts contributing to the recent rapid range expansion of the malaria vector Anopheles stephensi
Anopheles stephensi, the principal urban vector of malaria in South Asia, was first detected in Sri Lanka in Mannar island in 2017, with an origin in India (Figure 1) [47,48]. Anopheles stephensi was later observed to undergo preimaginal development in BW of up to 3.5 gL-1 in cement water storage tanks and domestic wells in different locations within Jaffna city (Figure 1) as well as Mannar Island [47-49]. It has been suggested that its adaptation to similar BW habitats in in neighboring coast of Tamil Nadu state in South India, and the movement of refugees and combatants in boats during the 1983-2009 civil war in Sri Lanka, as well as monsoonal winds across the 64-137 km-wide separating Palk strait (Figure 1), facilitated this expansion in the range of An. stephensi [49]. Anopheles stephensi also recently expanded its range west-wards to the Arabian Peninsula and after that to Northeast Africa with coastal zones playing a prominent role in this process [43,49]. Further expansion in the range of An. stephensi to West and East Africa was confirmed in 2023. It seems plausible that, as in Sri Lanka, civil wars in Yemen, Eritrea, Ethiopia, Sudan and Somalia accompanied by the (i) collapse of societal infrastructure, governments and vector control programs, (ii) movement of refugees, and (iii) adaptation to salinity, wells and cement water storage tanks, played a major role in the spread of An. stephensi and the consequent increased malaria transmission in the Arabian Peninsula and Africa[43].
Figure 5: Representative TBRF and LD IgM and IgG IBs with five sera from patients with LD-like symptoms. Sera of both patients in lanes 1 and 2 were only positive in TBRF IBs. Serum in lane 3 was positive in both TBRF and LD IBs. Serum in lane 4 was negative in both IBs. Serum in lane 5 was only positive in LD IBs. The detection of either IgM or IgG anti-bodies as shown in Figure 5 provides greater sensitivity than detecting either IgM or IgG alone. The different LDB and RFB antigens used in the LD and TBRF IBs are explained elsewhere [57,58]. Reproduced with permission under the creative commons license from [58].
Larval source reduction was an important component of efforts to control or eradicate malaria in the past but this no longer considered to be the case by the WHO [43]. However, the adaptation of FW malaria vectors to undergo preimaginal development in BW show the need for applying larval control measures to BW preimaginal habitats for controlling malaria in coastal areas [12, 28-30, 46-49]. This can also decrease malaria transmission inland because BW-developing vectors can act as bridging vectors in adjoining inland locations [26].
The larvivorous guppy Poecilia reticulata has been shown to prevent the development of An. stephensi in BW and FW domestic wells and cement water storage tanks [49]. Oreochromis mossambicus, a larvivorous tilapia fish, was effective against Anopheles and Aedes larvae in water storage tanks [50]. Larvae of the mosquito Culex fuscanus, that are able to develop in up to 10 gL-1 BW, were found to be successful predators of Aedes and Anopheles larvae [51]. The potential of these and other biological agents to control the preimaginal development of Anopheles malaria vectors and Aedes arboviral vectors (section 2) in potable FW as well as BW habitats merits further consideration. It is suggested that greater awareness of relevant scientific findings, facilitation of epidemiological studies to further evaluate the findings and promotion of malaria control measures in coastal areas by pertinent national and international malaria institutions are needed. However, reducing the impact of wars and movement of refugees on malaria control is a more difficult-to-address societal problem.
Laboratory tests supporting the clinical diagnosis and differentiation of Lyme disease and tick-borne relapsing fever
The pathogen causing Lyme Disease (LD) was first identified in ticks in 1982, and then in human patients in the US in 1983, to be a spirochete bacterium later named Borrelia burgdorferi. Borrelia burgdorferi sensu stricto (Bbss) is the principal species responsible for LD in the US [4,52]. Other species of the genus Borrelia termed Lyme Disease Borreliae (LDB), or alternatively Borrelia burgdorferi sensu lato, are now known to cause LD in many temperate zone countries. Some clinical manifestations of LD are shared with Tick-Borne Relapsing Fever (TBRF) caused by a different group of Borrelia species termed Relapsing Fever Borreliae (RFB), and indeed non-borrelial tick-borne diseases. TBRF is prevalent in both tropical and temperate climates in many parts of the world [4,53].
In the absence of distinct pathognomonic features, other than erythema migrans at the site of a tick bite which often goes unnoticed, a diagnosis of LD relies heavily on laboratory confirmation of infection. Because LDB are often present at low concentrations in blood, the detection of antibodies in patient sera has become the preferred laboratory diagnostic method [54,55]. The recommendation from the US National Conference on Serologic Diagnosis of Lyme Disease meeting in 1994 of a Standard Two-Tier Test (STTT) for the serological diagnosis of LD was an important diagnostic advance. The first tier of the STTT is an Enzyme Immunoassay (EIA) or Immunofluorescence Assay (IFA) on whole Bbss cell anti-gens, followed by a second-tier confirmatory Western Blot (WB) on whole Bbss cell lysates for sera that give positive or equivocal results in the first-tier test [55]. The rationale for the STTT was that the first tier EIA or IFA was highly sensitive but inadequately specific, while the second tier WB was highly specific for detecting serum antibodies to LDB. Drawbacks of STTTs include the need for a cumbersome WB procedure and variable sensitivity for detecting infections with LDB species other than Bbss, both of which can be overcome by using relevant recombinant proteins as target anti-gens. As a result, the CDC has been approving Modified Two-Tier Tests (MTTTs) amenable to machine reading of results in the US since 2019 [56].
Line immunoblot tests for the diagnosis and differentiation of tick-borne Lyme disease and relapsing fever borreliosis
Line Immunoblot Tests (IBs) based on purified recombinant proteins applied as lines on blotting media strips have been used to support the diagnosis of LD in European laboratories from the beginning of the 21st century. LD IB tests used potential single tier tests had comparable sensitivity and specificity to STTTs and were also able to detect antibodies against common European LDB species [4,57]. LD IBs tests have recently been developed together with analogous TBRF IB tests that detect RFB [4,58]. The use of TBRF IBs to test patients identified with LD-like symptoms in Ukraine, Australia and the US strongly suggested the many of them had TBRF and not LD. The parallel use of LD and TBRF IBs, as illustrated in Figure 5, can therefore be expected to be more reliable for diagnosing tick-borne borreliosis worldwide [4,58].
The CDC estimated, based on commercial insurance claims data, that approximately 476,000 persons were treated every year for LD in the US during the period 2010-2018 [59]. The number LD cases in Western Europe was estimated to be >200,000 per year and increasing every year [60]. The CDC reported 483 cases of TBRF during the period 1990-2011 in the US mainly confined to the western states, contrasting with other findings that suggest a greater incidence and wider distribution within the US. Recent nucleic acid sequence-based analysis showed TBRF to be a significant cause of acute febrile illness in patients suspected to have malaria in Senegal. Many such patients in many malaria-endemic countries would have been inappropriately treated with antimalarials whether malaria infections were confirmed or not by examination of stained blood smears. The use of TBRF IBs can be helpful in providing more definitive diagnosis and appropriate treatment, without the need for more sophisticated nucleic acid sequencing facilities, in this situation.
Many myths about LD propagated by the print, broadcast and internet media have long compromised its diagnosis and treatment. LD and TBRF in their early stages are readily amenable to antibiotic treatment. However, symptoms and pathology in chronic LD are complex and some cases of chronic LD are difficult to treat. Recent findings discussed here suggest a need to re-evaluate the incidence of LD and TBRF in the US and other countries. This would involve promoting further research to verify recent findings and greater coordination between scientific communities working on the diagnosis of LD and TBRF, by relevant national institutions like the CDC in the US.
SARS-CoV-2 infectivity and COVID-19 vaccines
The impact of vaccination during the COVID-19 pandemic: The WHO estimated that 774 million cases of COVID-19 had caused 7 million deaths worldwide up to the end of 2023. Vaccines against COVID-19 were rapidly developed after COVID-19 was first identified to provide the urgently needed initial immunity in the global population to combat the pandemic. One of the first to complete clinical trials was the University of Oxford-Astra Zeneca ChAdOx1 nCoV-19 vaccine utilising a recombinant chimpanzee adenovirus vector expressing the spike protein of SARS-CoV-2 as an immunogen. This vaccine was offered for use on a not-for-profit basis for the duration of the pandemic, and also licensed to be manufactured inexpensively by the Serum Institute of India.
During the first year of COVID-19 vaccination from December 2020 to December 2021, 55.9% of the global population have been estimated to have received one dose of a vaccine, 45.5% two doses, and 4.3% a third booster immunization. Based on officially reported COVID-19 deaths, 14·4 million (95% confidence interval 13·7-15·9), and alternatively the more reliable excess mortality data, 19·8 million (95% interval 19·1-20·4) deaths were estimated to have been averted by vaccination in 185 countries over this one-year period. Similar findings were reported at the country level from the US and Israel. Vaccination ameliorated the serious effects of the spread SARS-CoV2 infections among per-sons who had not previously had COVID-19, serving to enhance population immunity that minimized severe disease and mortality. Thus, COVID vaccines had a major beneficial impact on the early course of the pandemic and they continue to be used to boost immunity in elderly and immunocompromised persons at the present time. More lives would have been saved if resource-constrained countries had better early access to vaccines, some governments had not delayed beginning vaccinations or been more transparent about the local COVID-19 situation, and more people had not resisted receiving the vaccine due to cultural perceptions and misconceptions spread through the print, broadcast and internet media.
Differential susceptibility of population groups to SARS-CoV-2 infection: Increased susceptibility to infection by SARS-CoV-2 and consequent higher mortality during the temperate zone winter in persons with an ancestry in warm tropical countries, was widely attributed to socio-economic factors but not a likely weaker early immune response in the upper respiratory tract for which there is now increasing evidence. Timely recognition such likely genetic variations could have led to the implementation of appropriate public health and clinical measures that might have reduced infections, morbidity and mortality during the COVID-19 pandemic among vulnerable immigrant communities living in temperate zone countries.
Media-propagated misinformation on COVID-19 vaccines and delays in administering vaccines in some countries in-creased morbidity and mortality worldwide. Further investigations into varying SARS-CoV-2 infectivity among different population groups may be useful for minimizing morbidity and mortality in COVID-19 and other acute respiratory infections. It is suggested that these issues need to be addressed at the appropriate national and international levels.
Some less-well understood societal shortcomings that constrained the control of COVID-19, dengue, malaria, and tick-borne borreliosis, have been highlighted in this article. These probably caused some avoidable morbidity and mortality. There are two overarching societal drawbacks: (i) The need for greater cognizance of scientific advances and followed by timely initiation of appropriate responses on the part of pertinent national and authorities. More extensive investigations and scientific discussion may be needed to fully evaluate the relevant scientific findings cited here but this also needs to be facilitated by national and international institutions with relevant responsibilities; (ii) misleading in-formation in the print, broadcast and internet media, and the potential dangers of advanced computer technology in facilitating such misinformation. The general issue of misinformation in science, its consequences and the steps needed to address it, were prominently highlighted recently. Misinformation also negatively impacts the control of other infectious diseases. For example, misperceptions and misinformation spread through social internet media have led to reduced vaccine uptake not only for COVID-19 as discussed here but also measles. This has resulted in widespread resurgence of measles in many countries.
This research received no external funding.
Not applicable.
Not applicable.
All data supporting the conclusions of this article are included with in the article.
The author is affiliated to IDFISH and IGeneX which develop and apply diagnostic techniques for COVID-19, malaria and tick-borne diseases.