ISSN: 2320-2459
ISSN: 2320-2459
Department of Physics,Brahmdevdada Mane Institute of Technology, Solapur 413002, Maharashtra,India.
1Department of Physics,Shivaji University, Kolhapur, Maharashtra,India.
Received date: 23/03/2013 Revised date: 04/04/2013 Accepted date: 07/04/2013
Visit for more related articles at Research & Reviews: Journal of Pure and Applied Physics
Thin films of MnO2 are well suited for Electrochromic Photo-Voltaic (ECPV) effect and superconducting phenomenon (such as solar cells) due to its electrical and optical properties. MnO2 thin films were prepared on glass substrate using a simple and low-cost chemical spray pyrolysis technique at different substrate temperature. The MnO2 films were characterized by X-ray diffraction (XRD), transmittance and ultraviolet-visible (UV-VIS) optical spectroscopy. The XRD pattern reveals that MnO2 films possess a cubic structure. The transmittance activity of the sample was carried out by optical absorption studies. The indirect band gap of the material increases with increase in temperature. The dynamic of ion exchange was studied with CV, CA and CE. The maximum colouration efficiency observed is 17 cm2/C.
Manganese oxide, Spray pyrolysis, XRD, Band gap
Manganese oxide (MnO2) is a transition metal oxide. It has many opto-electronic applications. It is black in color and is used as electrode materials[1, 2], rechargeable batteries, catalysts, sensors [3], electrochemical capacitors [4,5], magnetoelectronic devices [6].MnO2 of different structures are depositedusing several techniques such as sol-gel [6], thermalevaporation in vacuum [10], MOCVD [10]. The MnO2 was used as a substrate in the synthesis of magnetic oxide perovskite compounds, which have a variety of electrical and magnetic properties like metal-insulator transistors and a colossal magneto resistance [7-9]. MnO2 of different structures are deposited using several techniques such as sol-gel [6], MOCVD [10], thermal evaporation in vacuum [11], spray pyrolysis [12].Manganeseoxide of different structure (MnO, Mn2O3, MnO2 andMn3O4) is usually prepared by varying calculationscondition of starting chemical precursor bulk or film. They can also be prepared each other by varying thetemperature and atmosphere (vacuum or air, oxygen, hydrogen etc.,) of the calcinations [11]. MnO2is preparedin the form of thin films on glass substrate by chemicalspray pyrolysis technique and their structural, opticalproperties are discussed. (Fig.1.1)
Experimental procedure
MnO2 thin films were grown on glass substrate using a typical spray pyrolysis technique. The spraying solution was prepared by dissolving appropriate quantity of precursorpowder (manganese acetate) in methanolat room temperature according to the equation,
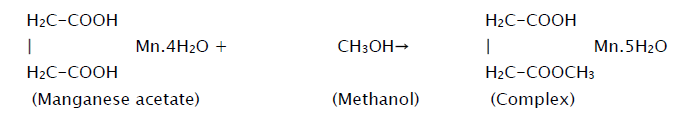
This solution is then atomized by compressed air at the pressure of 1 kg/cm2 on to the ultrasonically cleaned glass substrates. The sprayed droplets undergo solvent evaporation, solute condensation and thermal decomposition thereby resulting in the formation of manganese oxide thin film. The chemical reaction that takes place is given by the equation,
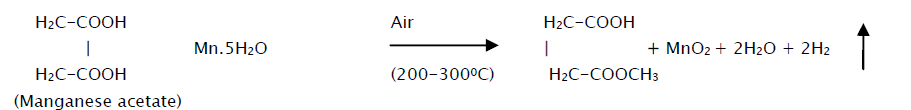
The substrate temperature was maintained at 200oC, 250 ºC and 300 ºC (± 2 ºC) through a thermocouple as a sensor for temperature controller. The spray rate was kept constant at 12cc/min. The carrier gas flow rate was kept constant at 15 lit/min.
The MnO2 films were subjected to X-ray diffraction technique to investigate the structural properties using anX-ray diffractometer (Philips PW1710) with CuKα radiation (λ = 1.5406 Å). Transmittances versus wavelengthmeasurements were made using UV-VIS- NIR spectrophotometer (Hitachi model 330).
Structural analysis
The structural elucidation of MnO2 film was shown in Fig. 1.2. The structural identification of MnO2 thin films deposited at various substrate temperatures from manganese acetate was carried out with X-ray diffraction. (The diffraction pattern obtained for samples T1, T2, T3 are as shown in Fig. 1.2.) The observed XRD patterns were compared with the JCPDS data [12]. The observed‘d’ values agree well with the standard‘d’ values and it was observed that all samples exhibit peaks corresponding to (104), (001), (311), (400) planes. The existence of shift in some peaks is due to internal strain existing in the crystalline due to the disproportionate array of the constituents [13]. The comparison with JCPDS data confirms the formation of Mn(OH)2 and MnO2. The standard and observed‘d’ values with corresponding (hkl) planes are listed in Table 1.
The samples T1 prepared at lower temperature 200oC consists of Mn(OH)2 phase indicating an incomplete thermal decomposition of manganese acetate. It was also observed that the prominence of MnO2 phase was found to improve with an increase in deposition temperature. The films prepared at 250oC consists of Mn(OH)2& λ- MnO2 and the films prepared at 300oC consists of λ- MnO2 films.
Transmittance analysis
The films were further analyzed by optical absorption studies as well as transmittance study. The variation of optical absorption density ‘αt’ with the wavelength ‘λ’ for the films were carried in the wavelength range 400 to 800 nm with UV spectrometer. This optical data were further analyzed to calculate the band gap energy of MnO2 samples using the relation,
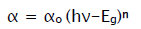
Where αo-constant, hν-photon energy, Eg - band gap energy
n= 1/2 for direct allowed transition
n = 2 for indirect allowed transition
Fig.1.3 shows the variation of (αhν)2 Vs (hν) for all samples and fig.1.4 shows the variation of (αhν)1/2 Vs (hν). The extrapolation of the straight line portions of the plots (αhν)2 Vs (hν) to zero absorption coefficient gives the values of direct band gap energy and extrapolation of the plots (αhν)1/2 Vs (hν) to gives the values of indirect band gap energy. It is found that indirect band gap of the samples increases with increase in temperature. The direct band gap energy for the samples at 250oC is minimum as compared to other samples.
MnO2 thin films were prepared using by spray pyrolysis technique. The structural analysis revealed the cubical nature of the film; it is also observed that the prominence of MnO2 phase was found to improve with an increase in deposition temperature. The indirect band gap energy of the material increases with increase in temperature.
The authors would like express sincere thanks to all staff members of the physics department, Shivaji University, Kolhapur, Brahmdevdada Mane Institute of Technology, Solapur and University for their cooperation. The authors also acknowledge the help received from the staff of University science and Instrumentation Centre (USIC) and Common Facility Centre (CFC), Shivaji University, Kolhapur.