ISSN: 2320-2459
ISSN: 2320-2459
J Glory1, PS Naidu2*, N Jayamadhuri3, and K Ravindra Prasad1
1Department of Physics, VSU PG Centre, Kavali – 524201, Andhra Pradesh, India.
2Department of Physics, JB Degree& PG College, Kavali – 524201, Andhra Pradesh, India.
3NBKR Institute of Science and Technology, Vidyanagar–524413, Andhra Pradesh, India.
Received date: 15/03/2013 Revised date: 04/04/2013 Accepted date: 09/04/2013
Visit for more related articles at Research & Reviews: Journal of Pure and Applied Physics
Ultrasonic velocity, density and viscosity have been measured using the standard techinques in the binary mixtures of benzyl benzoate (1) and 1-octanol (2) and isophorone (2). In the light of the excess parameters like excess adiabatic compressibility, excess internal pressure, excess enthalpy, excess activation energy etc. computed from the measured data, intermolecular interactions are estimated as strong AB interactions in both the mixtures. It is also observed that all the theories except FLT and VANDAEL have a sharp edge in predicting the velocities theoretically
Molecular interactions, binary mixture, benzyl benzoate, isophorone, adiabatic compressibility.
Through several binary mixtures of benzyl benzoate[1,2,3,4,5,6,7] have been studied ultrasonically, still the investigations are going on. In view of the importance medicinally, the behaviour of benzyl benzoate in cyclic unsaturated ketone, isophorone and a high alkanol, 1-°Ctanol, has been ultrasonically studied in the present investigation. Benzyl benzoate is an insect repellant and used as a solubilizing agent, in oily injections and medicine for scabbies. 1-°Ctanol, a fattyalcolhol, is used in the manufacture of esters used in perfumes and flavouring, in pharmaceutical industry and also in controlling essential tremors and other types of involuntary neurological tremors. Isophorone, which is an α β – unsaturated cyclic ketone, is used as solvent in some printing inks, paintings, lacquers, adhesives and some pesticides. It is also an ingradient in wood preservations and floor sealants. Ultrasonic vel°City, density and viscosity have been measured experimentally at three temperatures in the two binary mixtures of benzoate. From knowledge of excess thermodynamic / acoustic parameters computed, the molecular interactions have been investigated / estimated. Also a theoretical evaluation of vel°Cities has been attempted successfully. Almost all the theories except FLT and VANDAEL agree well with the experiment in both the mixtures. And mostly strong AB interactions are suggested in both the binary mixtures at all temperatures.
Ultrasonical vel°City has been measured using a single crystal variable path interferometer working at 2 MHz with an accuracy of + 0 .05%. Density and viscosity have been measured employing a double stem capillary type pyknometer and Ostwald viscometer with accuracies of 2 parts in 105 and + 0.1% respectively. All the chemicals used here are of analar / fine grade. Weights are taken using a single pan electronic balance with an accuracy of+ 0.05 mg. Temperature is maintained to within +0.01K employing an electronically controlled Thermostatic water bath. For standardizing the measurements, triply distilled water has been chosen as a reference liquid.
Theoretical Aspects
Employing the standard theories -- FLT due to JACOBSON, CFT due to SCHAAFFS, NOMOTO, VANDAEL, JUNJIEand JOUYBAN – ACREE,
ultrasonic vel°City can be evaluated theoretically.
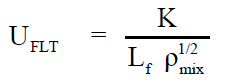 (1)
(1)
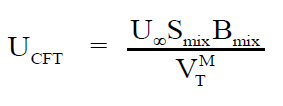 (2)
(2)
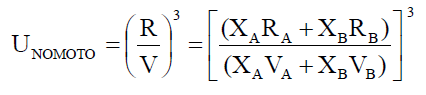 (3)
(3)
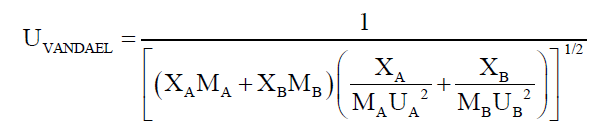 (4)
(4)
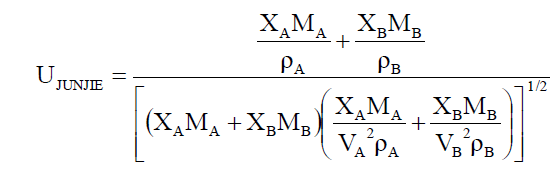 (5)
(5)
Vel°City(U) in the mixture due to JOUYBAN – ACREE is given by
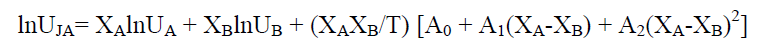 (6)
(6)
The thermodynamic/acoustic and other related parameters can be computed from the following relations.
 (7)
(7)
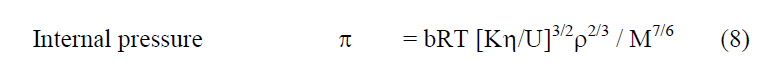
Free volume 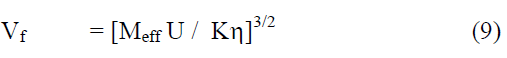
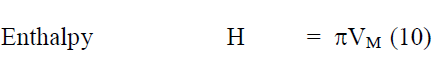
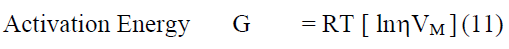
All the excess parameters are calculated using the following relation
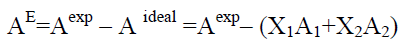 (12)
(12)
All the quantities used in the above equations have their usual meaning as explained elsewhere[8].
Ultrasonic vel°City, density and viscosity have been measured experimentally for the two binary systems 1) benzyl benzoate + 1-°Ctanol and 2) benzyl benzoate + isophorone at three temperatures 30,40 and 50 0C. The results and discussion have been made systematically and separately for the two systems and a comparison is made at the end. All the pure liquids are highly polar (dipole moment =3.9D, 2.0D and 3.96D for Benzyl benzoate, 1-°Ctanol and isophorone respectively).
Benzyl benzoate + 1 – °Ctanol
Ultrasonic vel°City, density and viscosity measured experimentally at three temperatures 30, 40 and 50°C have been presented in Table 1. Variation of experimental vel°City with concentration has been shown in Fig.1 along with the theoretically evaluated vel°Cities. Vel°City increases from °Ctanol to benzyl benzoate at all the three temperatures. From the maximum percentage deviations i.e., -5.26, 2.25, 1.56, - 3.94, 1.11 and 0.39 in FLT, CFT, NOMOTO, VANDAEL, JUNJIE and JOUYBAN – ACREE respectively, it may be inferred that JOUYBAN – ACREE agrees very well while FLT and VANDAEL show large deviations.
From the measured values of viscosity, density and vel°City, thermodynamic and other related parameters like adiabatic compressibility (β), internal pressure (π), enthalpy (H), activation energy (G), free length (Lf) etc., are computed and presented in Table 2. It may be seen that β, π and Lf decrease regularly while H also decreases but not regularly. Molar volume (Vm) and G increase from °Ctanol to benzoate at all temperatures. But to assess the nature of chemical reactions / intermolecular interactions, excess parameters would be of much use and hence the computed excess parameters are shown in Figs. 2-7. LfEand βE are both negative upto high concentration ( ~ 0.8m) and thereafter positive at all temperatures. ÃÂßE and HE are also negative throughout with a minimum nearly at equimolar concentrations (~0.5m) - less negative after 0.5m. ηE and GE are also negative throughout. It may be abserved that almost all excess parameters are negative and behave similarly with temperature. From the negative excess parameters, it may be indicated that there exists strong interamolecular interactions in the binary mixture and small variation in the parameters at high temperatures may be due to the small relative ass°Ciation between the molecules of 1-°Ctanol also. From ηEand GE, negative throughout the composition range at all temperatures, exothermic reactions appear to be predominant.
Benzyl benzoate + isophorone
Isophorone is a cyclic unsaturated ketone which readily reacts with benzoate, an ester. As discussed from Fig.8, vel°City increases from isophorone to benzoate monotonously and most linearly at all temperatures. Vel°City, density and viscosity in this system are shown in Table 3. Maximum percentage deviations observed in various theories are – 4.38, 0.98, -0.17, -4.26, -0.44 and -0.08 in FLT, CFT, NOMOTO, VANDEAL, JUNJIE and JOUYBAN – ACREE respectively at 30°C. Except FLT and VANDAEL, all the other theories agree reasonably with the experiment (<1% deviation). From the measured data experimentally, thermodynamic / other related parameters are computed and shownin Table 4. β and Lf show decreasing trend from isophorone to benzoate while the others π, Vm, H and G increase. The excess parameters computed for understanding the molecular interactions effectively have been shown in Figs. 9-14. It can be seen that βE, LfE, πE and HE are all negative throughout at all temperatures while at 50°C GE and ηE are negative and positive at 40°C throughout the concentration range. At 300C,both negative and positive are noticed.
In this system also, it is clearly indicated that strong intermolecular interactions of the type AB besides dipole – dipole interactions are predominant at all temperatures. At 30°C, both endothermic and exothermic type of chemical reactions are suggested which transform into endothermic at 40°C.
At this point of journey, a comparison of our results with those of other researchers in similar systems is desirable and hence is presented hereunder.
In the binary mixtures of benzyl benzoate with alcohols (methanol, ethanol, 1-propoanol, 1-butanol), strong AB interactions besides dipole – dipole interactions are estimated and the strength of the interaction decreased with the chain length. In the ketone systems (MEK & BMK) and acetophenone system also, mostly strong intermolecular interactions are suggested through weak interactions at some concentrations are not totally ruled out. The nature of chemical reaction is endothermic in the systems with alcohols and the two ketones (MEK & BMK) at all temperatures. In acetophenone system, endothermic type of reactions is also predominant at some concentrations and temperature. Other studies in benzyl benzoate and methyl benzoate9and other systems[10, 11]also reveal similar type of interactions from the variation of excess thermodynamic parameters.
Referring to the above literature on benzyl benzoate systems, our results and discussion are in conformity. In the mixture with the unsaturated cyclic ketone, exothermic at 30°C and endothermic at high temperatures and totally exothermic at all temperatures in the higher alcohol system are noticed. In both the systems, strong AB interactions besides dipole – dipole interactions are suggested. The highly polar nature of the constituents - the benzoate and isophorone may be the reason for (nearly equal) stronger interactions in the binary mixture compared to the other mixture.
The authors thank SVU and VSU authorities for providing the necessary facilities for carrying out this work. One of the authors (JG) acknowledges the sanction of RGNF (Senior) by the UGC.