ISSN: 2320-2459
ISSN: 2320-2459
D Madhavi Latha1, P Pardhasaradhi2, PV Datta Prasad3*, D Venkat Rao4 and VGKM Pisipati2,3
1Department of Physics, National Institute of Technology, Warangal, 506 004, India
2Liquid Crystal Research Centre, Department of Electronics and Communication Engineering, Koneru Lakshmaiah University, Vaddeswaram, 522 502, India
3SD Techs, Machilipatnam, 521 001, India
4SV College of Engineering, Nellore-524 316, India
Received date: 05/05/2014; Revised date: 10/06/2014; Accepted date: 18/06/2014
Visit for more related articles at Research & Reviews: Journal of Pure and Applied Physics
Thermodynamic parameters; Beyer’s nonlinearity parameter; Liquid crystal compounds; PBnA compounds, Moelwyn-Hughes Parameter, Sound velocity
It successfully finds one, it reports the found region. If it fails, the “signal graph matching” group will take over the The nonlinearity parameter B/A defined as the ratio of the quadratic term to that of the linear term in the equation of state, expressed as a Taylor series expansion, is an important parameter in non linear acoustics [1]. It has been used to represent the nonlinear effects such as wave form distortion, formation of higher harmonics and acoustic attenuation when a sound wave propagates through a medium. Researchers found that the acoustic nonlinearity parameter B/A was not only the parameter describing the degree of nonlinearity, but also the parameter which may provide some structural and state information of media.
Sehgal [2] has reported a set of relations involving the non linearity parameter, B/A for the determination of the molecular properties such as internal pressure, cohesive energy, effective Van der Walls constant, distance of closest approach of molecules, diffusion coefficient and rotational correlation time of pure liquids such as water, alcohols and fluorocarbons. Moreover, the nonlinearity parameter (B/A) has the potential importance in biological applications which provide information on the state of the tissues.
There have been many measurements of B/A in liquids from sound velocity by many workers utilizing different potentials [3]. These studies have shown that the value of B/A varies from 5 to 12. In some cases the extreme values of 2 and 13 are also reported [3]. Further, it is stated that this parameter can also be estimated from the thermal expansion coefficient, α obtained from the density data and from the sound velocity results, utilizing the expressions of Sharma [4], Hartmann et al [5] and Ballou [6]. The data on B/A in liquid crystals is meager and the present work provides an opportunity for the study of its variation in a liquid crystal with temperature in different phases as well as at the phase transformation. Further, systematically its variation with the alkyl and alkoxy chain numbers in a homologous series is studied. In the present manuscript, the non-linearity parameter (B/A) is estimated in the case of p-n-phenylbenzylidene- p-alkylanilines with alkyl chain number, n = 4 to 10, 12, 14,1 6 and18.
Theory
There are a number of reports in literature describing in detail the theoretical and empirical approach for the estimation of B/A from α and u respectively. However, for ready reference, the manuscript presents the relevant expressions that are used in the present study. The non-linearity parameter, B/A is given as [7]
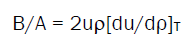 (1)
(1)
General formalism for B/A in terms of the acoustical parameters (Kand K’) for liquids and polymers has been made using Moelwyn-Hughes parameter (C1), isobaric acoustic parameter (K) and the isothermal acoustic parameter (K”) [8] obtained from the thermal expansion coefficient, α, and from the sound velocity, u respectively. The expressions for B/A from density are given as [7]
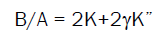 (2)
(2)
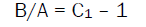 (3)
(3)
The molecular radius (Mr) for the LC molecule can be obtained from density and the expression is given below [9].
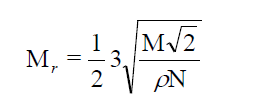 (4)
(4)
where M is the molecular weight.
The p-n-phenylbenzylidene-p-alkylanilines with alkyl chain number, n=4 to 10, 12, 14, 16 and 18 are chosen to evaluate, the Beyer’s nonlinearity parameter (B/A) molecular radius, (Mr). The experimental data required for the estimation was taken from the literature [10,11]. The estimated values of the above parameters are presented in tables 1 and 2 respectively.
The Nonlinearity parameter (B/A) calculated using eq. (2) and eq. (3) are presented in Table 1. The B/A value almost remains constant in a particular phase except at the vicinity of the phase transition where it shows a peak and the peak magnitude depends on α. The magnitude of B/A is slightly smaller in LC phases compared to that in isotropic phase,
The values of B/A estimated from eq. 3 are slightly lower to those obtained from eq.2. The results in table 1 reveal that the values obtained in isotropic phase are slightly higher compared to those obtained in liquid crystalline phases. Further, it is found that there is no regualr variation B/A with the n value and at the same it cannot be conclude conclusively about odd-even effect.
The molecular radius (Mr) is calculated using the eq. (4) and their values in different liquid crystalline phases in all the compounds is depicted in table 2. The variation of molecular radius with temperature follows as that of molar volume in all the cases. It exhibits jumps at the phase transition temperature and it is found that the molecular radius, Mr increases with the increase of alkyl chain number. From the results it is found that the core radius is 4.49 Å and the increment for methylene unit is 0.056 Å. In the case of alkoxy benzoic acids [12] it is found that these values are 3.58 Å and 0.084 Å respectively.
D.Madhavi Latha acknowledges the financial support of DST-WOS-A through the grant No.SR/WOS-A/PS-05/2010. The authors P. Pardhasaradhi and Dr.V.G.K.M.Pisipati, express thanks to The Head, ECE Dept. and the management of K.L.University, Vaddeswaram 522 502, India for providing facilities. This work is supported by the Department of Science and Technology (Grant No: SR/S2/CMP-0071/2008). Dr.P.V.Datta Prasad expresses their deep appreciation to the management of the SD Techs, Machilipatnam, India for providing assistance and D.Venkat Rao thanks the management of S V College of Engineering, Nellore-524 316, India for allowing him to work on this aspect.