ISSN: 2321-6204
ISSN: 2321-6204
I Sani*
Department of Biochemistry, Kebbi State University of Science and Technology, Aliero, P.M.B. 1144, Birnin Kebbi, Kebbi State, Nigeria.
Received date: 15/11/2013 Accepted date: 26/12/2013
Visit for more related articles at Research & Reviews: Journal of Food and Dairy Technology
Due to the abundance of Mangifera indica L. (mango) in virtually every part of Nigeria and considering its high consumption, this work was carried out to determine the potential applications of the waste seed kernel by investigating the physicochemical characteristics of its extracted oil content. The oil content was extracted using Soxhlet apparatus, and the physicochemical characterization was done using standard methods. The physicochemical parameters of the extracted oil were as follows: free fatty acids (78.9 11.25%), peroxide value (65.7 1.16 meqH2O2), acid value (5.8 0.25 mg KOH), iodine value (39.6 0.73 gI2/100g), saponification value (142.9 2.07 mgKOH/g) and specific gravity (2.18 0.01). The Mangifera indica L. seed kernel oil was observed to be golden yellow in colour, solid at room temperature and has a pleasant odour when fresh and unpleasant when rancid. Mangifera indica L. seed kernel has very low oil content of 8.27%, the oil contain unsaturated and saturated fatty acids. Justification of the use of the seed kernel oil for food, and cosmetic was expatiated.
Mangifera indica L. Seed, Seed Kernel, Oil Extraction, Physicochemical Characterization, Food
Mangifera indica L. (Mango) belongs to the genus Mangifera of the family Anacardiaceae. The genus Mangifera contains several species that bear edible fruits. Most of the fruit trees that are commonly known as mangoes belong to the species Mangifera indica L. [1, 2]. Mango trees are tropical fruit bearing plants, which thrive well in Asia and Africa.
Mangifera indica L. is an evergreen tree in the anacardiaceaea family that grows to a height of 10-15m, dome shaped with dense foliage, typically heavy branched from a stout trunk. The leaves are spirally arranged on branches, linear-oblong, lanceolate-elliptical, pointed at both ends, the leaf blades mostly about 25cm long and 8cm wide, sometimes much larger, reddish and thinly flaccid when first formed and release an aromatic odour when crushed. The fruit is a well known large drupe, but shows a great variation in shape and size. It contains a thick yellow pulp, single seed and thick yellowish-red skin when ripe. The seed is solitary, ovoid or oblong, encased in a hard, compressed fibrous endocarp [3].
Ripe mango fruit is considered to be invigorating and freshening. The juice is restorative, tonic and used as food. The seeds are usually discarded after consumption of the fleshy part of the fruits.
Collection and Preparation of Sample
Ripe mango fruits were purchased from Kali village of Aliero Local Government Area of Kebbi State in April, 2013. The peels and pulp were removed by washing in clean water, while the seeds were separated and cracked manually to remove the shells and hulls. The kernels were well dried in an open air at room temperature for 72 h, then ground using pestle and mortar. The powder i.e. the mango kernel meal (MKM) was used for the oil extraction.
Oil Extraction
The routine extraction of 50g of the ground MKM was conducted in a Soxhlet extractor according to the method described by [4] using n-hexane (boiling between 40–60 oC) for six hours. The oil was obtained after the solvent was removed under reduced temperature and pressure and refluxing at 70 ºC to remove excess solvent used in the oil. The process of cooling and heating was repeated until sufficient quantity of the oil was obtained. The Extracted oil was stored in a freezer at -2 ºC for subsequent physicochemical analysis.
Determination of Oil Yield
The total amount of oil obtained from the mango seed kernel by the complete distillation of the solvent on the heating mantle was transferred into a measuring cylinder. The measuring cylinder was then placed over water bath for 2-3 h in order to completely evaporate the little solvent present in the oil.
The percentage oil yield was calculated as follows:
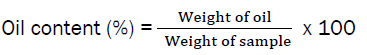
Physicochemical Analysis
The physicochemical properties (free fatty acids, saponification value, acid value, peroxide value, specific gravity and iodine value) of the oil were carried out using the method described by [5]. All determinations were done in triplicate.
Determination of Free Fatty Acids
2.0g of the seed kernel oil was transferred into 250cm3 Erlenmeyer flask followed by the addition of 100cm3 of ethanol and 2cm3 of phenolphthalein indicator. The contents were properly mixed and titrated against 0.04 M NaOH. The shaking continued until a slight pink colour which was steady for about 30 seconds was observed which signified the end point.
The expression of free fatty acids (FFA) is as follows:
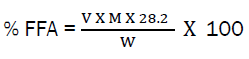
Where; % FFA = Percentage free fatty acid (oleic acid), V = Average volume of NaOH used (cm3), M = molarity of NaOH, 28.2g/mol = Molecular weight of oleic acid, W = weight of oil
Determination of Specific Gravity
A 50ml measuring cylinder was thoroughly washed with detergent water and petroleum ether then dried and weighed. 10cm3 of the oil was transferred into the cylinder and weighed. The weight of the oil was determined by subtracting the weight of the cylinder from the weight of oil and cylinder.
The specific gravity of the oil was obtained by the expression below
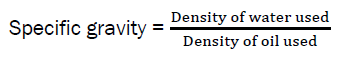
Determination of Peroxide Value
2.0g of the oil was weight into a clean dry flask and 22cm3 of a mixture of 12cm3of chloroform and 10cm3of acetic acid was added. Then followed by addition of 0.5cm3of potassium iodide.
The flask was closed and allowed to stay with constant shaking for 1 minute. 30cm3 of distilled water was then added and titrated against 0.1M of sodium thiosulphate (Na2S2O3) solution until an initial yellow colour disappeared and a faint blue colour appeared. 0.5cm3 of starch indicator was added quickly with continuous titration until there was a sudden disappearance of the blue color which signifies the end point.
The peroxide value is often reported as the number of ml of 0.02M Sodium thiosulphate per gram of sample. Thus peroxide value is obtained by the use of the following expression:
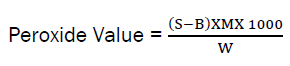
Where; Peroxide value = mEq of peroxide per 100g of sample, S = Sample titre value (cm3), B = Blank titre value (cm3), M = molarity ofNa2S2O3 solution (mEq/cm3), 1000 = Conversion of units (g/kg), W = Weight of oil sample
Determination of Acid Value
25cm3 of 5% ethanol was measured into a conical flask and boiled in a water bath in order to remove dissolved gasses. 2.5g of the oil was transferred into the 25cm3 of hot ethanol with continuous heating until the mixture boiled. After which, few drops of 1% phenolphthalein indicator were added and titrated against 0.1M KOH. The shaking continued until a permanent pink color appeared which signified the end point.
Acid value is expressed as follow:
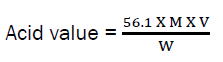
Where; M = Concentration of KOH, V = Titre value (cm3), 56.1 = molecular weight of KOH W = weight of oil sample
Determination of Iodine Value
0.4g of the oil was weighed into a conical flask and then dissolved by the addition of 20cm3 of carbon tetrachloride. After which, 25cm3 of wij’s reagent was added with the help of a safety pipette in a flame chamber. A stopper was inserted and the mixture was swirled vigorously before it was then placed in a dark room for duration of 2.5 h.
At the end of this period, 20cm3 of potassium iodide and 125cm3 of distilled water was added with the use of a measuring cylinder. The resulting solution was then titrated with 0.1M Sodium thiosulphate solution until the initial yellow colour almost disappeared. A few drops of 1% starch indicator was then added with few drops of thiosulphate added wisely as the titration continued with rigorous shaking until the blue coloration disappeared. The same procedure was carried out for the blank.
The calculative expression for iodine value is as follows:
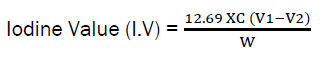
Where; C = Concentration of Sodium thiosulphate used, V1 = Volume of sodium thiosulphate used for blank, V2 = Volume of sodium thiosulphate used for test, W = Weight of Sample.
Determination of Saponification Value
2.0g of the oil was added into a clean dried flask containing 25ml of the ethanolic potassium hydroxide solution. A reflux condenser was attached and the flask was heated in boiling water for 1 h with continuous shaking to ensure complete dissolution of the sample. Then cooled and 1ml of phenolphthalein (1%) solution was added and titrated against 0.5M HCl until a pink coloration was observed which signified the end point.
The saponification value was calculated by the use of the expression below:
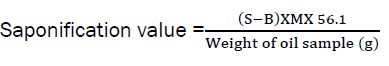
Where; S = Sample titre value (ml), B = Blank titre value (ml), M = Molarity of the HCl, 56.1 = molecular weight of KOH
The chemical properties of the Mangifera indica L. seed kernel oil are presented in Table 1, while the physical properties are presented in Table 2.
Free fatty acid content is the amount of free acids present per gram of the sample. The percentage of free fatty acids obtained from the result is 78.9 11.25, this shows that mango seed kernel oil is of low quality because it has high free fatty acids. High percentage of free fatty acids in crude oil is undesirable because they result in high losses of neutral oil during refining. In crude fat, free fatty acids estimate the amount of oil that will be lost during refining steps to remove fatty acids [6], indicating that the oil is not good for consumption. This could be as a result of impurities that could cause the hydrolysis of the ester linkage by increasing the free fatty acid level. They may be formed through hydrolysis or in the advanced stages of oxidation. An excessive amount of free fatty acids lower the smoke point of oil and cause popping of the oil during cooking. High quality oils are low in free fatty acids [7]. The free fatty acid value of this work is very high compared to other work earlier reported [8] which was (4.48 ± 0.44 ). This could be as a result of difference in climatic conditions of their geographical locations.
Peroxide value is the measure of oxidative rancidity of oil. Oxidative rancidity is the addition of oxygen across the double bonds in unsaturated fatty acids in the presence of enzymes or certain chemical compounds. High peroxide values are associated with higher rate of rancidity. The value obtained in this work indicates that the oil has high chance of becoming rancid, and the value is also higher than that obtained and reported earlier; 0.32 ± 0.12mEq H2O2 [8]. This means that this oil sample has higher chance of becoming rancid.
Acid value serves as an indicator for edibility of oil. It is the mg KOH required to neutralize the free fatty acid in 1g of oil. The acid value is used to measure the extent at which glycerides in oil are decomposed by lipases and other actions such as light and heat and that its determination is often used as general indication of the condition and edibility of oils [9]. In general, the lower the acid value the more its acceptability for edibility purpose [10]. The acid value of this oil sample is acceptable for edible purposes since it is slightly low.
Iodine value is a measure of the degree of unsaturation of oil. The lower the iodine value, the more saturated the oil since a higher iodine number indicates high unsaturation. A highly saturated fatty acid level is confirmed to be of benefit in terms of storage ability when compared to more unsaturated oils. The iodine value is also an index for assessing the ability of the oil to be rancid. The value obtained from this study indicates that the oil contain appreciable level of unsaturated fatty acid present especially oleic acid. Thus, all storage procedures should ensure protection of the oil from oxidative deterioration. The value also agrees with the one earlier reported [8].
Saponification value indicates the size or nature of fatty acid chains esterified to glycerol. It gives information about the character of the fatty acids of the oil and the solubility of their soaps in water. The higher the saponification value of fat free from moisture and unsaponifiable matter, the more the solubility of the soap produced from it. The oil sample analyzed has saponification number of 142.9 ± 2.07. Saponification value is inversely proportional to the molecular weight of the glycerides on the oil. The extract can also be used in the production of lecithin which is used in the manufacture of margarine [11].
The oil extraction from mango seed kernel was achieved and the physicochemical characteristics of the oil were determined. The research has shown that the mango seed yields very small amount of oil (8.27%), hence it cannot be used industrially. Some of the chemical parameters indicated the edibility of the oil but cannot be kept for long period of time.